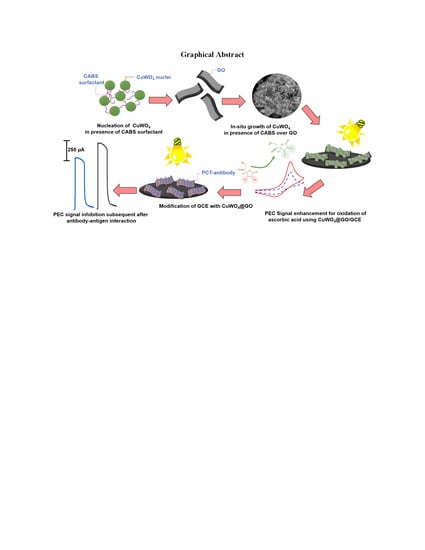
In Situ Growth of CuWO4 Nanospheres over Graphene Oxide for Photoelectrochemical (PEC) Immunosensing of Clinical Biomarker
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Situ Growth of CuWO4 Nanospheres over Graphene Oxide (rGO)
2.2. Developing a PEC Immunosensing Platform
2.3. PCT Detection from Simulated Blood Plasma
3. Results and Discussions
3.1. Characterization of CuWO4@rGO Hybrid
3.2. Photoelectrochemical Performance of CuWO4@rGO Hybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Qiao, X.; Liu, J.; Xia, F.; Tian, D.; Zhou, C. A dual-signals response electrochemiluminescence immunosensor based on PTC-DEPA/KCC-1 NCs for detection of procalcitonin. Sens. Actuators B Chem. 2018, 267, 525–532. [Google Scholar] [CrossRef]
- Jimeno, A.; García-Velasco, A.; del Val, O.; González-Billalabeitia, E.; Hernando, S.; Hernández, R.; Sánchez-Muñoz, A.; López-Martín, A.; Durán, I.; Robles, L. Assessment of procalcitonin as a diagnostic and prognostic marker in patients with solid tumors and febrile neutropenia. Cancer 2004, 100, 2462–2469. [Google Scholar] [CrossRef]
- Kaur, K.; Mahajan, R.; Tanwar, A. A novel marker procalcitonin may help stem the antibiotic overuse in emergency setting. Int. J. Appl. Basic Med. Res. 2013, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hu, Q.; Yu, X.; Wang, L. Ultrasensitive electrochemical immunosensor for procalcitonin with signal enhancement based on zinc nanoparticles functionalized ordered mesoporous carbon-silica nanocomposites. Sens. Actuators B Chem. 2018, 258, 238–245. [Google Scholar] [CrossRef]
- Fang, Y.S.; Wang, H.Y.; Wang, L.S.; Wang, J.F. Electrochemical immunoassay for procalcitonin antigen detection based on signal amplification strategy of multiple nanocomposites. Biosens. Bioelectron. 2014, 51, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lei, J.; Ju, H. Principles and applications of photoelectrochemical sensing strategies based on biofunctionalized nanostructures. Biosens. Bioelectron. 2017, 96, 8–16. [Google Scholar] [CrossRef]
- Soomro, R.A.; Hallam, K.R.; Ibupoto, Z.H.; Tahira, A.; Sherazi, S.T.H.; Memon, S.S.; Willander, M. Amino acid assisted growth of CuO nanostructures and their potential application in electrochemical sensing of organophosphate pesticide. Electrochim. Acta 2016, 190, 972–979. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, B.; Han, Q.; Lei, Y.; Wu, D.; Ren, X.; Wei, Q. Electrochemical immunosensor for ochratoxin A detection based on Au octahedron plasmonic colloidosomes. Anal. Chim. Acta 2018, 1032, 114–121. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, N.; Ali, A.; Wei, Q.; Wu, D.; Ren, X. Electrochemical ultrasensitive detection of cardiac troponin I using covalent organic frameworks for signal amplification. Biosens. Bioelectron. 2018, 119, 176–181. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhuo, Y.; Yuan, R.; Chai, Y.Q. An amplified electrochemical immunosensor based on in situ-produced 1-naphthol as electroactive substance and graphene oxide and Pt nanoparticles functionalized CeO2 nanocomposites as signal enhancer. Biosens. Bioelectron. 2015, 69, 321–327. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, F.; Feng, J.; Li, M.; Chen, L.; Dong, Y. Ultrasensitive electrochemical immunosensor for quantitative detection of SCCA using Co3O4@ CeO2-Au@ Pt nanocomposite as enzyme-mimetic labels. Biosens. Bioelectron. 2017, 92, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Köszegi, T. Immunoluminometric detection of human procalcitonin. J. Biochem. Biophys. Methods 2002, 53, 157–164. [Google Scholar] [CrossRef]
- Kremmer, E.; Meyer, K.; Grässer, F.A.; Flatley, A.; Kösters, M.; Luppa, P.B.; Krämer, P.M. A new strategy for the development of monoclonal antibodies for the determination of human procalcitonin in serum samples. Anal. Bioanal. Chem. 2012, 402, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, T.; Wu, D.; Yan, T.; Pang, X.; Du, B.; Lou, W.; Wei, Q. Increased electrocatalyzed performance through high content potassium doped graphene matrix and aptamer tri infinite amplification labels strategy: Highly sensitive for matrix metalloproteinases-2 detection. Biosens. Bioelectron. 2017, 94, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Soomro, R.A.; Kalwar, N.H.; Avci, A.; Pehlivan, E.; Hallam, K.R.; Willander, M. In-situ growth of NiWO4 saw-blade-like nanostructures and their application in photo-electrochemical (PEC) immunosensor systems designed for the detection of neuron-specific enolase. Biosens. Bioelectron. 2019, 141, 111331. [Google Scholar] [CrossRef] [PubMed]
- Soomro, R.A.; Sherazi, S.H.; Memon, N.; Shah, M.; Kalwar, N.; Hallam, K.R.; Shah, A. Synthesis of air stable copper nanoparticles and their use in catalysis. Adv. Mater. Lett. 2014, 5, 191–198. [Google Scholar] [CrossRef]
- Soomro, R.A.; Ibupoto, Z.H.; Abro, M.I.; Willander, M. Electrochemical sensing of glucose based on novel hedgehog-like NiO nanostructures. Sens. Actuators B Chem. 2015, 209, 966–974. [Google Scholar] [CrossRef]
- Soomro, R.A.; Nafady, A.; Ibupoto, Z.H.; Sherazi, S.T.H.; Willander, M.; Abro, M.I. Development of sensitive non-enzymatic glucose sensor using complex nanostructures of cobalt oxide. Mater. Sci. Semicond. Proc. 2015, 34, 373–381. [Google Scholar] [CrossRef]
- Abbas, M.W.; Soomro, R.A.; Kalwar, N.H.; Zahoor, M.; Avci, A.; Pehlivan, E.; Hallam, K.R.; Willander, M. Carbon quantum dot coated Fe3O4 hybrid composites for sensitive electrochemical detection of uric acid. Microchem. J. 2019, 146, 517–524. [Google Scholar] [CrossRef]
- Shakeel, M.; Zhang, X.; Yasin, G.; Arif, M.; Abbas, Z.; Zaman, U.; Li, B. Fabrication of Amorphous BiOCl/TiO2-C3N4 Heterostructure for Efficient Water Oxidation. ChemistrySelect 2019, 4, 8277–8282. [Google Scholar] [CrossRef]
- Hou, L.; Zhou, B.; Li, Y.; La, M. Progress in the Photoelectrochemical Biosensors for the Detection of MicroRNAs: A Review. Int. J. Electrochem. Sci. 2019, 14, 4453–4468. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Z.; Cheung, G.; Doughty, R.M.; Ballestas-Barrientos, A.R.; Hirmez, B.; Han, R.; Maschmeyer, T.; Osterloh, F.E. Role of surface states in photocatalytic oxygen evolution with CuWO4 Particles. J. Electrochem. Soc. 2019, 166, 3014–3019. [Google Scholar] [CrossRef]
- Ruiz-Fuertes, J.; Errandonea, D.; Segura, A.; Manjón, F.; Zhu, Z.; Tu, C. Growth, characterization, and high-pressure optical studies of CuWO4. High Press. Res. 2008, 28, 565–570. [Google Scholar] [CrossRef]
- Thiruppathi, M.; Kumar, J.V.; Vahini, M.; Ramalingan, C.; Nagarajan, E. A study on divergent functional properties of sphere-like CuWO4 anchored on 2D graphene oxide sheets towards the photocatalysis of ciprofloxacin and electrocatalysis of methanol. J. Mater. Sci. Mater. Electron. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, X.; Fan, D.; Kuang, X.; Wang, H.; Wu, D.; Wei, Q. Electrochemical procalcitonin immunoassay based on Au@Ag heterojunction nanorods as labels and CeO2-CuO nanorods as enhancer. Sens. Actuators B Chem. 2019, 297, 126800. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, G.; Chen, X.; Luo, F.; Jiang, D.; Huang, S.; Li, Y.; Pu, X. A novel strategy of procalcitonin detection based on multi-nanomaterials of single-walled carbon nanohorns–hollow Pt nanospheres/PAMAM as signal tags. RSC Adv. 2014, 4, 13934–13940. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, G.; Yuan, R.; Chen, X.; Luo, F.; Jiang, D.; Huang, S.; Li, Y.; Pu, X. Procalcitonin sensitive detection based on graphene–gold nanocomposite film sensor platform and single-walled carbon nanohorns/hollow Pt chains complex as signal tags. Biosens. Bioelectron. 2014, 60, 210–217. [Google Scholar] [CrossRef]






| Materials | Limit of Detection (LOD) (pg·mL−1) | Linear Range | Reference |
|---|---|---|---|
| Au@Ag heterojunction with CeO2-CuO | 0.17 | 0.5 pg·mL−1 to 50 ng·mL−1 | [25] |
| SWCNHs–HPtNPs/PAMAM hybrids | 1.74 | 10 pg·mL−1 to 20 ng·mL−1 | [26] |
| SWCNHs/ HPtCs hybrid | 0.43 | 1.00 pg·mL−1 to 20 ng·mL−1 | [27] |
| layer coated GC/ MWCNTs/CS/GA hybrid | 0.5 | 0.01 to 350 ng·mL−1 | [5] |
| PTC-DEPA/KCC-1 NCs | 0.017 | 0.05 pg mL−1 to 10 ng mL−1 | [1] |
| CuWO4@rGO | 0.15 | 10 pg·mL−1 to 50 ng·mL−1 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Z.; Soomro, R.A.; Kalwar, N.H.; Tunesi, M.; Willander, M.; Karakuş, S.; Kilislioğlu, A. In Situ Growth of CuWO4 Nanospheres over Graphene Oxide for Photoelectrochemical (PEC) Immunosensing of Clinical Biomarker. Sensors 2020, 20, 148. https://doi.org/10.3390/s20010148
Abbas Z, Soomro RA, Kalwar NH, Tunesi M, Willander M, Karakuş S, Kilislioğlu A. In Situ Growth of CuWO4 Nanospheres over Graphene Oxide for Photoelectrochemical (PEC) Immunosensing of Clinical Biomarker. Sensors. 2020; 20(1):148. https://doi.org/10.3390/s20010148
Chicago/Turabian StyleAbbas, Zaheer, Razium Ali Soomro, Nazar Hussain Kalwar, Mawada Tunesi, Magnus Willander, Selcan Karakuş, and Ayben Kilislioğlu. 2020. "In Situ Growth of CuWO4 Nanospheres over Graphene Oxide for Photoelectrochemical (PEC) Immunosensing of Clinical Biomarker" Sensors 20, no. 1: 148. https://doi.org/10.3390/s20010148