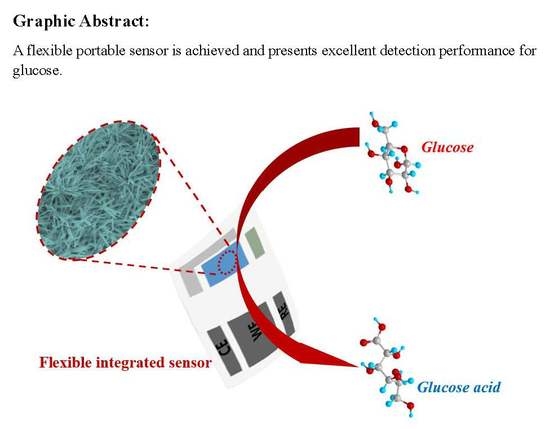
A Flexible Portable Glucose Sensor Based on Hierarchical Arrays of Au@Cu(OH)2 Nanograss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Cu/CFC
2.3. Preparation of Au@Cu(OH)2/CFC, Pt/CFC and Ag/AgCl/CFC
2.4. Preparation of the Flexible Integrated Sensor Device
2.5. Characterizations and Electrochemical Measurement
3. Results and Discussion
3.1. Characterizations of the Au@Cu(OH)2/CFC Sensor
3.2. Mechanism of Electrode Fabrication and Glucose Oxidation
3.3. Non-Enzymatic Glucose Electrochemical Performance of an Au@Cu(OH)2/CFC Sensor
3.4. Selectivity, Stability and Reproducibility of the Au@Cu(OH)2/CFC Sensor
3.5. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Baghayeri, M.; Amiri, A.; Farhadi, S. Development of non-enzymatic glucose sensor based on efficient loading Ag nanoparticles on functionalized carbon nanotubes. Sens. Actuators B Chem. 2016, 225, 354–362. [Google Scholar] [CrossRef]
- Jia, X.; Dong, S.; Wang, E. Engineering the bioelectrochemical interface using functional nanomaterials and microchip technique toward sensitive and portable electrochemical biosensors. Biosens. Bioelectron. 2016, 76, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, G.; Zhao, J.; Qiu, M.; Sun, P.; Fu, Y.; Han, D.; Cui, G. A portable micro-glucose sensor based on copper-based nanocomposite structure. New J. Chem. 2019, 43, 7806–7813. [Google Scholar] [CrossRef]
- Xu, M.; Song, Y.; Ye, Y.; Gong, C.; Shen, Y.; Wang, L.; Wang, L. A novel flexible electrochemical glucose sensor based on gold nanoparticles/polyaniline arrays/carbon cloth electrode. Sens. Actuators B Chem. 2017, 252, 1187–1193. [Google Scholar] [CrossRef]
- Chen, C.; Ran, R.; Yang, Z.; Lv, R.; Shen, W.; Kang, F.; Huang, Z. An efficient flexible electrochemical glucose sensor based on carbon nanotubes/carbonized silk fabrics decorated with Pt microspheres. Sens. Actuators B Chem. 2018, 256, 63–70. [Google Scholar] [CrossRef]
- Lin, S.; Feng, W.; Miao, X.; Zhang, X.; Chen, S. A flexible and highly sensitive nonenzymatic glucose sensor based on DVD-laser scribed graphene substrate. Biosens. Bioelectron. 2018, 110, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hwa, Y.; Soo, D.; Na, Y.; Kim, H.; Ho, D.; Ahn, S.; Yang, J.; Seok, W.; Seo, S. Flexible glucose sensor using CVD-grown graphene-based field effect transistor. Biosens. Bioelectron. 2012, 37, 82–87. [Google Scholar]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L. Polyaniline-Coated Electro-Etched Carbon Fiber Cloth Electrodes for Supercapacitors. J. Phys. Chem. C 2011, 115, 23584–23590. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.; Qiu, C.; Zhu, W.; Ma, H.; Chen, S.; Meng, O. Electrochemical sensing of glucose by carbon cloth-supported Co3O4/PbO2 core-shell nanorod arrays. Biosens. Bioelectron. 2014, 53, 200–206. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Wang, D.; Hasnain, M.; An, Q.; Han, D. Co3O4 nanostructures on flexible carbon cloth for crystal plane effect of nonenzymatic electrocatalysis for glucose. Biosens. Bioelectron. 2019, 123, 25–29. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Li, H.; Zhu, R.; Shao, Q.; Yang, S.; Xu, J. NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: Promising peroxidase mimetics for H2O2 and glucose detection. Biosens. Bioelectron. 2015, 64, 147–153. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, C.; Guo, Y.; Nie, G.; Xu, L. Peroxidase-like activity of apoferritin paired gold clusters for glucose detection. Biosens. Bioelectron. 2015, 64, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.H.; Ahn, S.J.; Lee, J.J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhong, H.; Kou, T.; Frenzel, J.; Eggeler, G.; Zhang, Z. Three-dimensional Cu foam-supported single crystalline mesoporous Cu2O nanothorn arrays for ultra-highly sensitive and efficient nonenzymatic detection of glucose. ACS Appl. Mater. Interfaces 2015, 7, 20215–20223. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhuang, J.; Yang, D.; Tang, D. Eggshell membrane-templated synthesis of 3D hierarchical porous Au networks for electrochemical nonenzymatic glucose sensor. Biosens. Bioelectron. 2017, 96, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, S.; Jeong, R.; Boo, H.; Park, J.; Chan, H.; Dong, T. Nonenzymatic continuous glucose monitoring in human whole blood using electrified nanoporous Pt. Biosens. Bioelectron. 2012, 31, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, S.; Han, B.; Wang, J.; Mao, R. Preparation and properties of enhanced bulk nanoporous coppers. Mater. Lett. 2012, 73, 136–138. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, L.; Wang, Z. Nonenzymatic amperometric determination of glucose by CuO nanocubes–graphene nanocomposite modified electrode. Bioelectrochemistry 2012, 88, 156–163. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Zhao, C.; Qian, X. One-step construction of hierarchical Ni(OH)2/RGO/Cu2O on Cu foil for ultra-sensitive non-enzymatic glucose and hydrogen peroxide detection. Sens. Actuators B Chem. 2018, 274, 163–171. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Su, L.; Bellagamba, M.; Zhang, H.; Lei, Y. Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosens. Bioelectron. 2010, 26, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Dhara, K.; Stanley, J.; Ramachandran, T.; Nair, B.G.; Satheesh, S.B. Pt-CuO nanoparticles decorated reduced graphene oxide for the fabrication of highly sensitive non-enzymatic disposable glucose sensor. Sens. Actuators B Chem. 2014, 195, 197–205. [Google Scholar] [CrossRef]
- Karuppiah, C.; Velmurugan, M.; Chen, S. A simple hydrothermal synthesis and fabrication of zinc oxide–copper oxide heterostructure for the sensitive determination of nonenzymatic glucose biosensor. Sens. Actuators B Chem. 2015, 221, 1299–1306. [Google Scholar] [CrossRef]
- Kong, C.; Lv, J.; Hu, X.; Zhao, N.; Liu, K.; Zhang, X.; Meng, G.; Yang, Z. Template-synthesis of hierarchical CuO nanoflowers constructed by ultrathin nanosheets and their application for non-enzymatic glucose detection. Mater. Lett. 2018, 219, 134–137. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, C.; Meng, X.; Chen, Z.; Li, Y.; Zhang, D.; Zhu, S. Design and fabrication of Ag-CuO nanoparticles on reduced graphene oxide for nonenzymatic detection of glucose. Sens. Actuators B Chem. 2018, 265, 435–442. [Google Scholar] [CrossRef]
- Dhara, K.; Ramachandran, T.; Nair, B.G.; Satheesh Babu, T.G. Single step synthesis of Au-CuO nanoparticles decorated reduced graphene oxide for high performance disposable nonenzymatic glucose sensor. J. Electroanal. Chem. 2015, 743, 1–9. [Google Scholar] [CrossRef]
- Luo, P.; Prabhu, S.V.; Baldwin, R.P. Constant Potential Amperometric Detection at a Copper-Based Electrode: Electrode Formation and Operation. Anal. Chem. 1990, 62, 752–755. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, X.; Shi, H.; Chen, J.; Zhang, F.; Song, W. Direct growth of vertically aligned arrays of Cu(OH )2 nanotubes for the electrochemical sensing of glucose. Sens. Actuators B Chem. 2013, 177, 445–452. [Google Scholar] [CrossRef]
- Shackery, I.; Patil, U.; Pezeshki, A.; Shinde, N.M.; Kang, S.; Im, S.; Chan, S. Copper Hydroxide Nanorods Decorated Porous Graphene Foam Electrodes for Non-enzymatic Glucose Sensing. Electrochim. Acta 2016, 191, 954–961. [Google Scholar] [CrossRef]
- You, T.; Niwa, O. Characterization and electrochemical properties of highly dispersed copper oxide/hydroxide nanoparticles in graphite-like carbon films prepared by RF sputtering method. Electrochem. Commun. 2002, 4, 468–471. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, L.; Zhang, W.; Gunasekaran, S. A highly sensitive non-enzymatic glucose sensor based on a simple two-step electrodeposition of cupric oxide (CuO) nanoparticles onto multi-walled carbon nanotube arrays. Talanta 2010, 82, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Nantaphol, S.; Watanabe, T.; Nomura, N.; Siangproh, W. Biosensors and Bioelectronics Bimetallic Pt–Au nanocatalysts electrochemically deposited on boron-doped diamond electrodes for nonenzymatic glucose detection. Biosens. Bioelectron. 2017, 98, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, L.; Zhou, C.; Xing, R.; Dai, Q.; Liu, D.; Song, H. Synthesis of graphene oxide based cuo nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 12928–12934. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Lv, J.; Tang, L.; Kong, C.; Song, X.; Yang, Z. Nanoparticle-aggregated CuO nanoellipsoids for high-performance non-enzymatic glucose detection. J. Mater. Chem. A 2014, 2, 10073–10080. [Google Scholar] [CrossRef]
- Luo, S.; Su, F.; Liu, C.; Li, J.; Liu, R.; Xiao, Y.; Li, Y.; Liu, X.; Cai, Q. A new method for fabricating a CuO/TiO2 nanotube arrays electrode and its application as a sensitive nonenzymatic glucose sensor. Talanta 2011, 86, 157–163. [Google Scholar] [CrossRef]
- Fan, G.; Sun, P.; Zhao, J.; Han, D.; De, D.L.N. Alleviating concentration polarization: A micro-three-electrode interdigitated glucose sensor based on nanoporous gold from a mild process. RSC Adv. 2019, 9, 10465–10472. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.; Lee, S.; Seo, M.; Dong, T. Recent advances in electrochemical non-enzymatic glucose sensors e A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Hoppe, M.; Ababii, N.; Postica, V.; Lupan, O.; Polonskyi, O.; Schütt, F.; Kaps, S.; Sukhodub, L.F.; Sontea, V.; Strunskus, T.; et al. (CuO-Cu2O)/ZnO:Al heterojunctions for volatile organic compound detection. Sens. Actuators B Chem. 2018, 255, 1362–1375. [Google Scholar] [CrossRef]
- Cui, S.; Liu, X.; Sun, Z.; Du, P. Noble Metal-Free Copper Hydroxide as an Active and Robust Electrocatalyst for Water Oxidation at Weakly Basic pH. ACS Sustain. Chem. Eng. 2016, 4, 2593–2600. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; St, R.; Smart, C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Mcintyre, N.S.; Cook, M.G. X-Ray Photoelectron Studies on Some Oxides and Hydroxides of Cobalt, Nickel and Copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Au, N.H.C.; Au, I.; Young, A.J.; Sauer, M.; Sato, A.; Foelske, A.; Reithofer, M.R.; Serpell, C.J.; Chin, M. One-step synthesis and XPS investigations of chiral NHC–Au(0)/Au(I) nanoparticles. Nanoscale 2019, 11, 8327–8333. [Google Scholar]
- Paixão, T.R.L.C.; Corbo, D.; Bertotti, M. Amperometric determination of ethanol in beverages at copper electrodes in alkaline medium. Anal. Chim. Acta 2002, 472, 123–131. [Google Scholar] [CrossRef]
- Wen-zhi, L.; You-qin, L. Preparation of nano-copper oxide modified glassy carbon electrode by a novel film plating/potential cycling method and its characterization. Sens. Actuators B Chem. 2009, 141, 147–153. [Google Scholar]
- Wu, H.; Cao, W.; Li, Y.; Liu, G.; Wen, Y.; Yang, H.; Yang, S. In situ growth of copper nanoparticles on multiwalled carbon nanotubes and their application as non-enzymatic glucose sensor materials. Electrochim. Acta 2010, 55, 3734–3740. [Google Scholar] [CrossRef]
- Wang, R.; Sun, P.; Wang, H.; Wang, X. Pulsed laser deposition of amorphous molybdenum disulfide films for efficient hydrogen evolution reaction. Electrochim. Acta 2017, 258, 876–882. [Google Scholar] [CrossRef]
- Xie, F.; Wu, H.; Mou, J.; Lin, D.; Xu, C.; Wu, C.; Sun, X. Ni3N@Ni-Ci nanoarray as a highly active and durable non-noble-metal electrocatalyst for water oxidation at near-neutral pH. J. Catal. 2017, 356, 165–172. [Google Scholar] [CrossRef]
- Qu, Y.; Medina, H.; Wang, S.; Wang, Y.; Chen, C.; Su, T.; Manikandan, A.; Wang, K.; Shih, Y.; Chang, J.; et al. Wafer Scale Phase-Engineered 1T- and 2H-MoSe2/MoCore–Shell 3D-Hierarchical Nanostructures toward Efficient Electrocatalytic Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 9831–9838. [Google Scholar] [CrossRef]
- Chen, H.; Sun, P.; Qiu, M.; Jiang, M.; Zhao, J.; Han, D.; Niu, L. Co-P decorated nanoporous copper framework for high performance flexible non-enzymatic glucose sensors. J. Electroanal. Chem. 2019, 841, 119–128. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Wang, H.; Wu, Y.; Lu, Z. High-performance hybrid electrode decorated by well-aligned nanograss arrays for glucose sensing. Biosens. Bioelectron. 2018, 102, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, J.; Zhang, S.; Wang, W.; Chen, Z. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles decorated carbon spheres. Sens. Actuators B Chem. 2015, 211, 385–391. [Google Scholar] [CrossRef]
- Kannan, P.; Maiyalagan, T.; Marsili, E.; Ghosh, S.; Niedziolka-jönsson, J.; Jönsson-niedziolka, M. Hierarchical 3-dimensional nickel–iron nanosheet arrays on carbon fiber paper as a novel electrode for non-enzymatic glucose sensing. Nanoscale 2015, 8, 843–855. [Google Scholar] [CrossRef]
- Liu, X.; Long, L.; Yang, W.; Chen, L.; Jia, J. Facilely electrodeposited coral-like copper micro-/nano-structure arrays with excellent performance in glucose sensing. Sens. Actuators B Chem. 2018, 266, 853–860. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, Q.; Jia, L.; Zhao, Q.; Jiang, J. Networked cobaltous phosphate decorated with nitrogen-doped reduced graphene oxide for non-enzymatic glucose sensing. Sens. Actuators B Chem. 2019, 283, 443–450. [Google Scholar] [CrossRef]






| Samples | Reference Values (mM) | Added Glucose (mM) | Au@Cu(OH)2/CFCc (mM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|
| Mulberry juice | 670.68a | 400.00 | 1030.83 ± 8.22 | 92.09 | 0.75 |
| 800.00 | 1439.60 ± 15.23 | 97.89 | 1.02 | ||
| Human serum | 5.4b | - | 5.37 | 99.44 | 0.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Sun, P.; Zhao, J.; Huo, L.; Cui, G. A Flexible Portable Glucose Sensor Based on Hierarchical Arrays of Au@Cu(OH)2 Nanograss. Sensors 2019, 19, 5055. https://doi.org/10.3390/s19225055
Jiang M, Sun P, Zhao J, Huo L, Cui G. A Flexible Portable Glucose Sensor Based on Hierarchical Arrays of Au@Cu(OH)2 Nanograss. Sensors. 2019; 19(22):5055. https://doi.org/10.3390/s19225055
Chicago/Turabian StyleJiang, Min, Peng Sun, Jie Zhao, Lihua Huo, and Guofeng Cui. 2019. "A Flexible Portable Glucose Sensor Based on Hierarchical Arrays of Au@Cu(OH)2 Nanograss" Sensors 19, no. 22: 5055. https://doi.org/10.3390/s19225055