Disposable Injection Molded Conductive Electrodes Modified with Antimony Film for the Electrochemical Determination of Trace Pb(II) and Cd(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Instrumentation
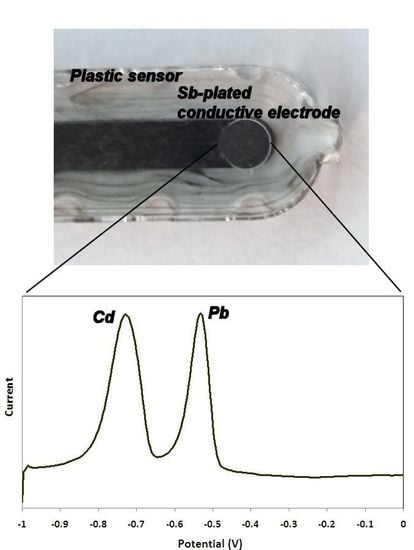
2.3. Fabrication of the Sensor
2.4. Experimental Procedure
3. Results and Discussion
3.1. Deposition of Sb on the Electrode
3.2. Selection of the Chemical and Instrumental Conditions
3.3. Metrological Features
3.4. Interferences
4. Application
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Bhattacharjee, T.; Goswami, M. Heavy Metals (As, Cd & Pb) Toxicity & Detection of These Metals in Ground Water Sample: A Review on Different Techniques. Int. J. Eng. Sci. Inven. 2018, 7, 12–21. [Google Scholar]
- Bulska, E.; Ruszczyńska, A. Analytical Techniques for Trace Element Determination. Phys. Sci. Rev. 2017, 2017–8002. [Google Scholar]
- Helaluddin, A.B.M.; Saadi Khalid, R.; Alaama, M.; Atif Abbas, S. Main Analytical Techniques Used for Elemental Analysis in Various Matrices. J. Pharm. Res. 2016, 15, 427–434. [Google Scholar] [CrossRef]
- Economou, A.; Kokkinos, C. Advances in stripping analysis of metals. In Electrochemical Strategies in Detection Science; Arrigan, D.W.M., Ed.; The Royal Society of Chemistry: Burlington House, London, UK, 2016; pp. 1–18. ISBN 978-1-84973-831-6. [Google Scholar]
- Wang, J. Stripping Analysis: Principles, Instrumentation and Applications; VCH: Deerfield Beach, FL, USA, 1985; ISBN 3-527-26192-3. [Google Scholar]
- Wang, J.; Tian, B.; Wang, J.; Lu, J.; Olsen, C.; Yarnitzky, C.; Olsen, K.; Hammerstrom, D.; Bennett, W. Stripping analysis into the 21st century: Faster, smaller, cheaper, simpler and better. Anal. Chim. Acta 1999, 385, 429–435. [Google Scholar] [CrossRef]
- Arino, C.; Serrano, N.; Díaz-Cruz, J.M.; Esteban, M. Voltammetric determination of metal ions beyond mercury electrodes. A review. Anal. Chim. Acta 2017, 990, 11–53. [Google Scholar] [CrossRef] [Green Version]
- Alves, G.M.S.; Rocha, L.S.; Soares, H.M.V.M. Multi-element determination of metals and metalloids in waters and wastewaters, at trace concentration level, using electroanalytical stripping methods with environmentally friendly mercury free-electrodes: A review. Talanta 2017, 175, 53–68. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.M.A.; Ogorevc, B. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef]
- Švancara, I.; Prior, C.; Hočevar, S.B.; Wang, J. A decade with bismuth-based electrodes in electroanalysis. Electroanal. 2010, 22, 1405–1420. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A. Stripping analysis at bismuth-based electrodes. Curr. Anal. Chem. 2008, 4, 183–190. [Google Scholar] [CrossRef]
- Hocevar, S.B.; Švancara, I.; Ogorevc, B.; Vytřas, K. Antimony film electrode for electrochemical stripping analysis. Anal. Chem. 2007, 79, 8639–8643. [Google Scholar] [CrossRef]
- Serrano, N.; Díaz-Cruz, J.M.; Arino, C.; Esteban, M. Antimony-based electrodes for analytical determinations. TrAC 2016, 77, 203–213. [Google Scholar] [CrossRef]
- Gharib Naseri, N.; Baldock, S.J.; Economou, A.; Goddard, N.J.; Fielden, P.R. Disposable Injection-Moulded Cell-on-a-Chip Microfluidic Devices with Integrated Conducting Polymer Electrodes for On-LineVoltammetric and Electrochemiluminescence Detection. Electroanalysis 2008, 20, 448–454. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Goddard, N.J.; Fielden, P.R.; Baldock, S.J. Determination of Pb(II) by sequential injection/stripping analysis at all-plastic electrochemical fluidic cells with integrated composite electrodes. Talanta 2016, 153, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Wilkins, S.J.; Marken, F.; Compton, R.G. Simultaneous Electrochemical Detection and Determination of Lead and Copper at Boron-Doped Diamond Film Electrodes. Electroanalysis 2002, 14, 262–272. [Google Scholar] [CrossRef]
- Sebez, B.; Ogorevc, B.; Hocevar, S.B.; Veber, M. Functioning of antimony film electrode in acid media under cyclic and anodic stripping voltammetry conditions. Anal. Chim. Acta 2013, 785, 43–49. [Google Scholar] [CrossRef]
- Guzsvany, V.; Nakajima, H.; Soh, N.; Nakano, K.; Imato, T. Antimony-film electrode for the determination of trace metals by sequential-injection analysis/anodic stripping voltammetry. Anal. Chim. Acta 2010, 658, 12–17. [Google Scholar] [CrossRef]
- Guzsvany, V.; Nakajima, H.; Soh, N.; Nakano, K.; Svancara, I.; Vytras, K.; Bjelica, L.; Imato, T. Anodic stripping voltammetry combined with sequential injection analysis for measurements of trace metal ions with bismuth- and antimony film electrodes under comparable conditions. Electroanalysis 2011, 23, 1593–1601. [Google Scholar] [CrossRef]
- Agra-Gutiérrez, C.; Hardcastle, J.L.; Ball, J.C.; Compton, R.G. Anodic stripping voltammetry of copper at insonated glassy carbon-based electrodes: Application to the determination of copper in beer. Analyst 1999, 124, 1053–1057. [Google Scholar] [CrossRef]
- Dragoe, D.; Spătaru, N.; Kawasaki, R.; Manivannan, A.; Spătaru, T.; Tryk, D.A.; Fujishima, A. Detection of trace levels of Pb2+ in tap water at boron-doped diamond electrodes with anodic stripping voltammetry. Electrochim. Acta 2006, 51, 2437–2441. [Google Scholar]
- Slavec, M.; Hocevar, S.B.; Baldrianova, L.; Tesarova, E.; Svancara, I.; Ogorevc, B.; Vytras, K. Antimony Film Microelectrode for Anodic Stripping Measurementof Cadmium(II), Lead(II) and Copper(II). Electroanalysis 2010, 22, 1617–1622. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Investigation of Copper(II) Interference on the Anodic StrippingVoltammetry of Lead(II) and Cadmium(II) at Bismuth Film Electrode. Electroanalysis 2013, 25, 2637–2644. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, S.; Hu, G.; Xu, H.; Wang, Y.; Yuan, Q. One-step synthesis of isoreticular metal–organic framework-8 derived hierarchical porous carbon and its application in differential pulse anodic stripping voltammetric determination of Pb(II). RSC Adv. 2015, 5, 77159–77167. [Google Scholar] [CrossRef]
- Serrini, G.; Muntau, H.; Colinet, E.; Griepink, B. Certification of the Contents of As, B, Cd, Cr, Co, Cu, Mn, Hg, Ni, Ti, V and Zn in a Natural Moroccan Phosphate Rock. Fresenius Z. Anal. Chem. 1983, 315, 691–695. [Google Scholar] [CrossRef]
- Maroulis, M.; Economou, A.; Voulgaropoulos, A. Determination of Cd and Pb in Phosphorites and PhosphateFertilizers by Means of a Portable Voltammetric Analyzer Based on“Virtual Instrumentation”. Electroanalysis 2007, 19, 2149–2154. [Google Scholar] [CrossRef]
- Voulgaropoulos, A.; Paneli, M.; Papaefstathiou, E.; Stavroulias, S. Comparative determinations of cadmium and lead in phosphorites dissolved in nitric acid and aqua regia using differential pulse anodic stripping voltammetry and atomic absorption spectrophotometry. Fres. J. Anal. Chem. 1991, 341, 568–569. [Google Scholar] [CrossRef]







| Calibration Equation | R 1 | LOD (μg L−1) 2 | |
|---|---|---|---|
| Cd(II) | Ip (μΑ) = (0.469 ± 0.004) CCd (μg L−1) + (0.347 ± 0.103) (μΑ) | 0.998 | 1.3 |
| Pb(II) | Ip (μΑ) = (0.482 ± 0.006) CPb (μg L−1) + (0.442 ± 0.152) (μΑ) | 0.997 | 0.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christidi, S.; Chrysostomou, A.; Economou, A.; Kokkinos, C.; Fielden, P.R.; Baldock, S.J.; Goddard, N.J. Disposable Injection Molded Conductive Electrodes Modified with Antimony Film for the Electrochemical Determination of Trace Pb(II) and Cd(II). Sensors 2019, 19, 4809. https://doi.org/10.3390/s19214809
Christidi S, Chrysostomou A, Economou A, Kokkinos C, Fielden PR, Baldock SJ, Goddard NJ. Disposable Injection Molded Conductive Electrodes Modified with Antimony Film for the Electrochemical Determination of Trace Pb(II) and Cd(II). Sensors. 2019; 19(21):4809. https://doi.org/10.3390/s19214809
Chicago/Turabian StyleChristidi, Savvina, Alexia Chrysostomou, Anastasios Economou, Christos Kokkinos, Peter R. Fielden, Sara J. Baldock, and Nicholas J. Goddard. 2019. "Disposable Injection Molded Conductive Electrodes Modified with Antimony Film for the Electrochemical Determination of Trace Pb(II) and Cd(II)" Sensors 19, no. 21: 4809. https://doi.org/10.3390/s19214809