An Efficient Electrochemical Sensor Driven by Hierarchical Hetero-Nanostructures Consisting of RuO2 Nanorods on WO3 Nanofibers for Detecting Biologically Relevant Molecules
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis of Hybrid Nanostructures of RuO2 Nanorods on Electrospun WO3 Nanofibers
3.2. Electrochemical Properties for Capacitive Behaviors of RuO2 NRs-WO3 NFs
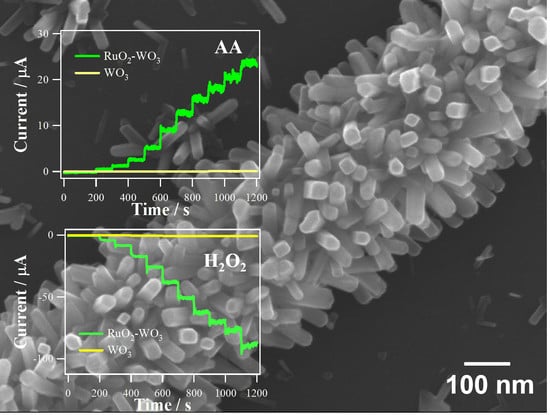
3.3. Applications to Electrochemical Sensing of AA and H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.L.; Choi, H.-A.; Lee, N.-S.; Son, B.; Kim, H.J.; Baik, J.M.; Lee, Y.; Lee, C.; Kim, M.H. RuO2–ReO3 composite nanofibers for efficient electrocatalytic responses. Phys. Chem. Chem. Phys. 2015, 17, 7435–7442. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Cho, Y.K.; Seok, J.; Lee, N.-S.; Son, B.; Lee, J.W.; Baik, J.M.; Lee, C.; Lee, Y.; Kim, M.H. Highly Branched RuO2 Nanoneedles on Electrospun TiO2 Nanofibers as an Efficient Electrocatalytic Platform. ACS Appl. Mater. Interfaces 2015, 7, 15321–15330. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Yang, Y.; Lee, N.-S.; Son, B.; Lee, Y.; Lee, C.; Kim, M.H. Electrospun RuO2–Co3O4 hybrid nanotubes for enhanced electrocatalytic activity. Mater. Lett. 2015, 139, 405–408. [Google Scholar] [CrossRef]
- Sugimoto, W.; Iwata, H.; Yokoshima, K.; Murakami, Y.; Takasu, Y. Proton and Electron Conductivity in Hydrous Ruthenium Oxides Evaluated by Electrochemical Impedance Spectroscopy: The Origin of Large Capacitance. J. Phys. Chem. B 2005, 109, 7330–7338. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.; Chang, K.-H.; Lin, M.-C.; Wu, Y.-T. Design and Tailoring of the Nanotubular Arrayed Architecture of Hydrous RuO2 for Next Generation Supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef]
- Zang, J.; Bao, S.-J.; Li, C.M.; Bian, H.; Cui, X.; Bao, Q.; Sun, C.Q.; Guo, J.; Lian, K. Well-Aligned Cone-Shaped Nanostructure of Polypyrrole/RuO2 and Its Electrochemical Supercapacitor. J. Phys. Chem. C 2008, 112, 14843–14847. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Yuan, G.; Cui, C.; Lu, L. A Symmetric RuO2/RuO2 Supercapacitor Operating at 1.6 V by Using a Neutral Aqueous Electrolyte. Electrochem. Solid-State Lett. 2012, 15, A60–A63. [Google Scholar] [CrossRef]
- Lee, J.-B.; Jeong, S.-Y.; Moon, W.-J.; Seong, T.-Y.; Ahn, H.-J. Preparation and characterization of electro-spun RuO2–Ag2O composite nanowires for electrochemical capacitors. J. Alloys Compd. 2011, 509, 4336–4340. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Zhang, X.-G. Preparation and electrochemical capacitance of RuO2/TiO2 nanotubes composites. Electrochim. Acta 2004, 49, 1957–1962. [Google Scholar]
- Wang, Y.-G.; Wang, Z.-D.; Xia, Y.-Y. An asymmetric supercapacitor using RuO2/TiO2 nanotube composite and activated carbon electrodes. Electrochim. Acta 2005, 50, 5641–5646. [Google Scholar] [CrossRef]
- Rack Ahn, Y.; Park, C.; Mu Jo, S.; Young Kim, D. Enhanced charge-discharge characteristics of RuO2 supercapacitors on heat-treated TiO2 nanorods. Appl. Phys. Lett. 2007, 90, 122106. [Google Scholar]
- Lokhande, C.; Park, B.-O.; Park, H.-S.; Jung, K.-D.; Joo, O.-S. Electrodeposition of TiO2 and RuO2 thin films for morphology-dependent applications. Ultramicroscopy 2005, 105, 267–274. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, Y.K.; Lee, C.; Kim, M.H.; Lee, Y. Real-time direct electrochemical sensing of ascorbic acid over rat liver tissues using RuO2 nanowires on electrospun TiO2 nanofibers. Biosens. Bioelectron. 2016, 77, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.L.; Ha, Y.; Lee, N.-S.; Kim, J.G.; Baik, J.M.; Lee, C.; Yoon, K.; Lee, Y.; Kim, M.H. Hybrid architecture of rhodium oxide nanofibers and ruthenium oxide nanowires for electrocatalysts. J. Alloys Compd. 2016, 663, 574–580. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J. Tungsten Oxides for Photocatalysis, Electrochemistry, and Phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef] [PubMed]
- Janáky, C.; Chanmanee, W.; Rajeshwar, K. On the Substantially Improved Photoelectrochemical Properties of Nanoporous WO3 Through Surface Decoration with RuO2. Electrocatalysis 2013, 4, 382–389. [Google Scholar] [CrossRef]
- Baruffaldi, C.; Cattarin, S.; Musiani, M. Deposition of non-stoichiometric tungsten oxides+MO2 composites (M = Ru or Ir) and study of their catalytic properties in hydrogen or oxygen evolution reactions. Electrochim. Acta 2003, 48, 3921–3927. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, Y.; Yu, J.; Yin, J.; Bai, X. High electrochemical activity from hybrid materials of electrospun tungsten oxide nanofibers and carbon black. J. Mater. Sci. 2012, 47, 6607–6613. [Google Scholar] [CrossRef]
- Wei, H.; Ding, D.; Yan, X.; Guo, J.; Shao, L.; Chen, H.; Sun, L.; Colorado, H.A.; Wei, S.; Guo, Z. Tungsten Trioxide/Zinc Tungstate Bilayers: Electrochromic Behaviors, Energy Storage and Electron Transfer. Electrochim. Acta 2014, 132, 58–66. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, E.; Kim, J.K.; Lee, C.W.; Lee, J. Development of high-performance supercapacitor electrodes using novel ordered mesoporous tungsten oxide materials with high electrical conductivity. Chem. Commun. 2011, 47, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Pu, Z.; Liu, Q.; Asiri, A.M.; Hu, J.; Sun, X. Tungsten nitride nanorods array grown on carbon cloth as an efficient hydrogen evolution cathode at all pH values. Electrochim. Acta 2015, 154, 345–351. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.-J.; Youn, D.-Y.; Kim, I.-D. Exhaled VOCs sensing properties of WO3 nanofibers functionalized by Pt and IrO2 nanoparticles for diagnosis of diabetes and halitosis. J. Electroceram. 2012, 29, 106–116. [Google Scholar] [CrossRef]
- Shim, J.; Lee, C.-R.; Lee, H.-K.; Lee, J.-S.; Cairns, E.J. Electrochemical characteristics of Pt–WO3/C and Pt–TiO2/C electrocatalysts in a polymer electrolyte fuel cell. J. Power Sources 2001, 102, 172–177. [Google Scholar] [CrossRef]
- Jayaraman, S.; Jaramillo, T.F.; Baeck, S.-H.; McFarland, E.W. Synthesis and Characterization of Pt–WO3 as Methanol Oxidation Catalysts for Fuel Cells. J. Phys. Chem. B 2005, 109, 22958–22966. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, B.; Karthik, V.; Karthikeyan, S.; Ravindranathan Thampi, K.; Bonard, J.M.; Viswanathan, B. Pt–WO3 supported on carbon nanotubes as possible anodes for direct methanol fuel cells. Fuel 2002, 81, 2177–2190. [Google Scholar] [CrossRef]
- Kuo, L.-M.; Chen, K.-N.; Chuang, Y.-L.; Chao, S. A Flexible pH-Sensing Structure Using WO3/IrO2 Junction with Al2O3 Encapsulation Layer. ECS Solid State Lett. 2013, 2, P28–P30. [Google Scholar] [CrossRef]
- Jeong, Y.U.; Manthiram, A. Amorphous Tungsten Oxide/Ruthenium Oxide Composites for Electrochemical Capacitors. J. Electrochem. Soc. 2001, 148, A189–A193. [Google Scholar] [CrossRef]
- Chen, K.Y.; Sun, Z.; Tseung, A.C.C. Preparation and Characterization of High-Performance Pt-Ru/WO3-C Anode Catalysts for the Oxidation of Impure Hydrogen. Electrochem. Solid-State Lett. 2000, 3, 10–12. [Google Scholar] [CrossRef]
- Yang, L.X.; Bock, C.; MacDougall, B.; Park, J. The role of the WOx ad-component to Pt and PtRU catalysts in the electrochemical CH3OH oxidation reaction. J. Appl. Electrochem. 2004, 34, 427–438. [Google Scholar] [CrossRef]
- Ruzgas, T.; Csöregi, E.; Emnéus, J.; Gorton, L.; Marko-Varga, G. Peroxidase-modified electrodes: Fundamentals and application. Anal. Chim. Acta 1996, 330, 123–138. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, Y.L.; Yu, A.; Lee, J.; Lee, S.C.; Lee, C.; Kim, M.H.; Lee, Y. Electrospun iridium oxide nanofibers for direct selective electrochemical detection of ascorbic acid. Sens. Actuators B 2014, 196, 480–488. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.W.; Ye, B.U.; Chun, S.H.; Joo, S.H.; Park, H.; Lee, H.; Jeong, H.Y.; Kim, M.H.; Baik, J.M. Structural Evolution of Chemically-Driven RuO2 Nanowires and 3-Dimensional Design for Photo-Catalytic Applications. Sci. Rep. 2015, 5, 11933. [Google Scholar] [CrossRef] [PubMed]
- Habazaki, H.; Hayashi, Y.; Konno, H. Characterization of electrodeposited WO3 films and its application to electrochemical wastewater treatment. Electrochim. Acta 2002, 47, 4181–4188. [Google Scholar] [CrossRef]
- Shi, J.; Allara, D.L. Characterization of High-Temperature Reactions at the BaO/W Interface. Langmuir 1996, 12, 5099–5108. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Karlsson, R.K.B.; Cornell, A.; Pettersson, L.G.M. Structural Changes in RuO2 during Electrochemical Hydrogen Evolution. J. Phys. Chem. C 2016, 120, 7094–7102. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.; Gao, Y.; Wu, J.; Tang, B.; Zhao, J. Amorphous nickel–boron and nickel–manganese–boron alloy as electrochemical pseudocapacitor materials. RSC Adv. 2014, 4, 27800–27804. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326. [Google Scholar] [CrossRef]
- Jo, A.; Kang, M.; Cha, A.; Jang, H.S.; Shim, J.H.; Lee, N.S.; Kim, M.H.; Lee, Y.; Lee, C. Nonenzymatic amperometric sensor for ascorbic acid based on hollow gold/ruthenium nanoshells. Anal. Chim. Acta 2014, 819, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.R.; Chatraei, F. Preparation and electrochemical characteristics of electrodeposited acetaminophen on ruthenium oxide nanoparticles and its role as a sensor for simultaneous determination of ascorbic acid, dopamine and N-acetyl-l-cysteine. Sens. Actuators B 2011, 160, 1450–1457. [Google Scholar] [CrossRef]
- Wu, J.; Suls, J.; Sansen, W. Amperometric determination of ascorbic acid on screen-printing ruthenium dioxide electrode. Electrochem. Commun. 2000, 2, 90–93. [Google Scholar] [CrossRef]
- Prakash, P.; Wei Ting, S.; Chen, S.-M. Amperometric and Impedimetric H2O2 Biosensor Based on Horseradish Peroxidase Covalently Immobilized at Ruthenium Oxide Nanoparticles Modified Electrode. Int. J. Electrochem. Sci. 2011, 6, 2688–2709. [Google Scholar]
- Anjalidevi, C.; Dharuman, V.; Shankara Narayanan, J. Non enzymatic hydrogen peroxide detection at ruthenium oxide–gold nano particle–Nafion modified electrode. Sens. Actuators B 2013, 182, 256–263. [Google Scholar] [CrossRef]







| Electrodes | Methods | Solutions | Potential /V | Sensitivity /μAmM−1 cm2 | Linear Range /μM |
|---|---|---|---|---|---|
| RuO2 NRs-WO3 NFs 1 | Amperometry | PBS (pH 7.4) | 0 | 171.7 | 5–2000 |
| RuO2-Co3O4 hybrid nanotubes 2 | Amperometry | PBS (pH 7.4) | 0.05 | 204 | ~500 |
| RuO2NWs-TiO2NFs 3 | Amperometry | PBS (pH 7.4) | 0.018 | 268.2 | 10–1500 |
| hAu-Ru nanoshells 4 | Amperometry | PBS (pH 7.4) | 0.05 | 426 | 5–2000 |
| AC-RuON-GCE 5 | DPV | PBS (pH 7.0) | −0.053 | 85.9 | 47–181.8 |
| Screen-printing RuO2 6 | Amperometry | PBS (pH 7.4) | 0.058 | 2.79 | 0–4000 |
| Electrodes | Methods | Solutions | Potential /V | Sensitivity /μA mM−1 cm−2 | Linear Range /μM |
|---|---|---|---|---|---|
| RuO2 NRs-WO3 NFs 1 | Amperometry | 0.1 M PBS | −0.2 | 619.7 | 5–2000 |
| RuO2-ReO3 (0.11) 2 | Amperometry | 0.1 M PBS | −0.2 | 667.8 | 0–5000 |
| RuO2NNs-TiO2 NRs 3 | Amperometry | 0.05M PBS | 0 | 53.8 | 1–1000 |
| RuO2 NWs-Rh2O3 NF 4 | Amperometry | 0.05 M PBS | 0.12 | 283.1 | 0–1000 |
| HRP/Chi-GAD/RuNPs 5 | Amperometry | Saturated PBS | −0.3 | 0.798 | 5090–15,000 |
| Nafion-RuO2-AuNP flim 6 | Amperometry | PBS | −0.4 | 15.44 | 0.001–30,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, Y.; Yu, A.; Jin, D.; Jo, A.; Lee, Y.; Kim, M.H.; Lee, C. An Efficient Electrochemical Sensor Driven by Hierarchical Hetero-Nanostructures Consisting of RuO2 Nanorods on WO3 Nanofibers for Detecting Biologically Relevant Molecules. Sensors 2019, 19, 3295. https://doi.org/10.3390/s19153295
Lee H, Kim Y, Yu A, Jin D, Jo A, Lee Y, Kim MH, Lee C. An Efficient Electrochemical Sensor Driven by Hierarchical Hetero-Nanostructures Consisting of RuO2 Nanorods on WO3 Nanofibers for Detecting Biologically Relevant Molecules. Sensors. 2019; 19(15):3295. https://doi.org/10.3390/s19153295
Chicago/Turabian StyleLee, Hyerim, Yeomin Kim, Areum Yu, Dasol Jin, Ara Jo, Youngmi Lee, Myung Hwa Kim, and Chongmok Lee. 2019. "An Efficient Electrochemical Sensor Driven by Hierarchical Hetero-Nanostructures Consisting of RuO2 Nanorods on WO3 Nanofibers for Detecting Biologically Relevant Molecules" Sensors 19, no. 15: 3295. https://doi.org/10.3390/s19153295