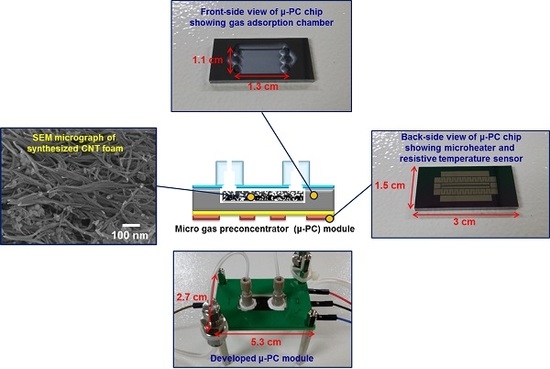
CNT Foam-Embedded Micro Gas Preconcentrator for Low-Concentration Ethane Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Principles and Requirements
2.2. Materials
Synthesis Method for CNT Foam
2.3. Microfabrication
2.4. Experimental Setup and Performance Test
2.4.1. Pressure-Drop Test of Gas-Adsorbing Materials
2.4.2. Preconcentration Test of the µ–PC Module
3. Results and Discussions
3.1. Pressure-Drop Test of Gas-Adsorbing Materials
3.2. Preconcentration Test of the µ–PC Module
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Amann, A.; CostelloBde, L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Camara, E.H.M.; Breuil, P.; Briand, D.; De Rooij, N.F.; Pijolat, C. A micro gas preconcentrator with improved performance for pollution monitoring and explosives detection. Anal. Chim. Acta 2011, 688, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skeldon, K.D.; Patterson, C.; Wyse, C.A.; Gibson, G.M.; Padgett, M.J.; Longbottom, C.; McMillan, L.C. The potential offered by real-time, high-sensitivity monitoring of ethane in breath and some pilot studies using optical spectroscopy. J. Opt. A Pure Appl. Opt. 2005, 7, S376. [Google Scholar] [CrossRef]
- Abbott, S.M.; Elder, J.B.; Španěl, P.; Smith, D. Quantification of acetonitrile in exhaled breath and urinary headspace using selected ion flow tube mass spectrometry. Int. J. Mass Spectrom. 2003, 228, 655–665. [Google Scholar] [CrossRef]
- Bloemen, K.; Hooyberghs, J.; Desager, K.; Witters, E.; Schoeters, G. Non-invasive biomarker sampling and analysis of the exhaled breath proteome. Proteom. Clin. Appl. 2009, 3, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Riess, U.; Tegtbur, U.; Fauck, C.; Fuhrmann, F.; Markewitz, D.; Salthammer, T. Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NOX in exhaled human breath. Anal. Chim. Acta 2010, 669, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Spinhirne, J.P.; Koziel, J.A.; Chirase, N.K. A device for non-invasive on-site sampling of cattle breath with solid-phase microextraction. Biosyst. Eng. 2003, 84, 239–246. [Google Scholar] [CrossRef]
- Ye, M.; Chien, P.J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.Y.; Burnham, E.L.; Moss, M.; Brown, L.A. Non-invasive evaluation of pulmonary glutathione in the exhaled breath condensate of otherwise healthy alcoholics. Respir. Med. 2008, 102, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.W.; Dweik, R.A. Applied breath analysis: An overview of the challenges and opportunities in developing and testing sensor technology for human health monitoring in aerospace and clinical applications. J. Breath Res. 2008, 2, 037020. [Google Scholar] [CrossRef] [PubMed]
- Kneepkens, C.F.; Lepage, G.; Roy, C.C. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic. Biol. Med. 1994, 17, 127–160. [Google Scholar] [CrossRef]
- Buszewski, B.; Kęsy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Bessa, V.; Darwiche, K.; Teschler, H.; Sommerwerck, U.; Rabis, T.; Baumbach, J.I.; Freitag, L. Detection of volatile organic compounds (VOCs) in exhaled breath of patients with chronic obstructive pulmonary disease (COPD) by ion mobility spectrometry. Int. J. Ion Mobil. Spectrom. 2011, 14, 7–13. [Google Scholar] [CrossRef]
- Kanoh, S.; Kobayashi, H.; Motoyoshi, K. Exhaled ethane: An in vivo biomarker of lipid peroxidation in interstitial lung diseases. Chest 2005, 128, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Beurden, W.J.C.; Dekhuijzen, P.N.R.; Smeenk, F.W.J.M. Exhaled biomarkers in COPD: Their potential role in diagnosis, treatment and prognosis. Monaldi Arch. Chest Dis. 2002, 57, 258–267. [Google Scholar] [PubMed]
- Righettoni, M.; Tricoli, A. Toward portable breath acetone analysis for diabetes detection. J. Breath Res. 2011, 5, 037109. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, T.; Hiyama, S.; Yamada, Y. A prototype portable breath acetone analyzer for monitoring fat loss. J. Breath Res. 2013, 7, 036005. [Google Scholar] [CrossRef] [PubMed]
- Wlodzimirow, K.A.; Abu-Hanna, A.; Schultz, M.J.; Maas, M.A.W.; Bos, L.D.J.; Sterk, P.J.; Knobel, H.H.; Soers, R.J.T.; Chamuleau, R.A. Exhaled breath analysis with electronic nose technology for detection of acute liver failure in rats. Biosens. Bioelectron. 2014, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Chang, H.; Zellers, E.T. Microfabricated gas chromatograph for the selective determination of trichloroethylene vapor at sub-parts-per-billion concentrations in complex mixtures. Anal. Chem. 2011, 83, 7198–7206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Burris, D.R.; Chang, H.; Bryant-Genevier, J.; Zellers, E.T. Microfabricated gas chromatograph for on-site determination of trichloroethylene in indoor air arising from vapor intrusion. Environ. Sci. Technol. 2012, 46, 6065–6072. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.R.; Manginell, P.; Adkins, D.R.; Kottenstette, R.J.; Wheeler, D.R.; Sokolowski, S.S.; Trudell, D.E.; Byrnes, J.E.; Okandan, M.; Bauer, J.M.; et al. Recent advancements in the gas-phase MicroChemLab. IEEE Sens. J. 2006, 6, 784–795. [Google Scholar]
- Lu, C.J.; Steinecker, W.H.; Tian, W.C.; Oborny, M.C.; Nichols, J.M.; Agah, M.; Potkay, J.A.; Chan, J.; Driscoll, H.K.L.; Sacks, R.D.; et al. First-generation hybrid MEMS gas chromatograph. Lab Chip 2005, 5, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Gràcia, I.; Ivanov, P.; Blanco, F.; Sabaté, N.; Vilanova, X.; Correig, X.; Fonseca, L.; Figueras, E.; Santander, J.; Cané, C. Sub-ppm gas sensor detection via spiral μ-preconcentrator. Sens. Actuators B Chem. 2008, 132, 149–154. [Google Scholar]
- Ruiz, A.M.; Gràcia, I.; Sabaté, N.; Ivanov, P.; Sànchez, A.; Duch, M.; Gerbolés, M.; Moreno, A.; Cané, C. Membrane-suspended microgrid as a gas preconcentrator for chromatographic applications. Sens. Actuators A. Phys. 2007, 135, 192–196. [Google Scholar] [CrossRef]
- Voiculescu, I.; McGill, R.A.; Zaghloul, M.E.; Mott, D.; Stepnowski, J.; Stepnowski, S.; Summers, H.; Nguyen, V.; Ross, S.; Walsh, K.; et al. Micropreconcentrator for enhanced trace detection of explosives and chemical agents. IEEE Sens. J. 2006, 6, 1094–1104. [Google Scholar] [CrossRef]
- Zheng, F.; Baldwin, D.L.; Fifield, L.S.; Anheier, N.C.; Aardahl, C.L.; Grate, J.W. Single-walled carbon nanotube paper as a sorbent for organic vapor preconcentration. Anal. Chem. 2006, 78, 2442–2446. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.C.; Chan, H.K.; Lu, C.J.; Pang, S.W.; Zellers, E.T. Multiple-stage microfabricated preconcentrator-focuser for micro gas chromatography system. J. Microelectromech. Syst. 2005, 14, 498–507. [Google Scholar] [CrossRef]
- Lahlou, H.; Sanchez, J.B.; Vilanova, X.; Berger, F.; Correig, X.; Fierro, V.; Celzard, A. Towards a GC-based microsystem for benzene and 1, 3 butadiene detection: Pre-concentrator characterization. Sens. Actuators B Chem. 2011, 156, 680–688. [Google Scholar] [CrossRef]
- Davis, C.E.; Ho, C.K.; Hughes, R.C.; Thomas, M.L. Enhanced detection of m-xylene using a preconcentrator with a chemiresistor sensor. Sens. Actuators B Chem. 2005, 104, 207–216. [Google Scholar] [CrossRef]
- Takada, S.; Nakai, T.; Thurakitseree, T.; Shiomi, J.; Maruyama, S.; Takagi, H.; Shuzo, M.; Delaunay, J.-J.; Yamada, I. Micro gas preconcentrator made of a film of single-walled carbon nanotubes. IEEJ Trans. Sens. Micromach. 2010, 130, 207–211. [Google Scholar] [CrossRef]
- Lahlou, H.; Leghrib, R.; Llobet, E.; Vilanova, X.; Correig, X. Development of a gas pre-concentrator based on carbon nanotubes for benzene detection. Procedia Eng. 2011, 25, 239–242. [Google Scholar] [CrossRef]
- Chae, M.S.; Kim, J.; Yoo, Y.K.; Kang, J.Y.; Lee, J.H.; Hwang, K.S. A micro-preconcentrator combined olfactory sensing system with a micromechanical cantilever sensor for detecting 2,4-dinitrotoluene gas vapor. Sensors 2015, 15, 18167–18177. [Google Scholar] [CrossRef] [PubMed]
- Alfeeli, B.; Cho, D.; Ashraf-Khorassani, M.; Taylor, L.T.; Agah, M. MEMS-based multi-inlet/outlet preconcentrator coated by inkjet printing of polymer adsorbents. Sens. Actuators B Chem. 2008, 133, 24–32. [Google Scholar] [CrossRef]
- Dow, A.B.A.; Lang, W. A micromachined preconcentrator for ethylene monitoring system. Sens. Actuators B Chem. 2010, 151, 304–307. [Google Scholar]
- Rydosz, A.; Maziarz, W.; Pisarkiewicz, T.; Domański, K.; Grabiec, P. A gas micropreconcentrator for low level acetone measurements. Microelectron. Reliab. 2012, 52, 2640–2646. [Google Scholar] [CrossRef]
- Vereb, H.; Alfeeli, B.; Dietrich, A.; Agah, M. Using MEMS-based preconcentrators to identify iron catalyzed lipid oxidation products in breath. In Proceedings of the IEEE Sensors Conference, University of Limerick, Limerick, Ireland, 28–31 October 2011; pp. 1237–1240. [Google Scholar]
- Kim, M.; Mitra, S. A microfabricated microconcentrator for sensors and gas chromatography. J. Chromatogr. A 2003, 996, 1–11. [Google Scholar] [CrossRef]
- Simoes, E.W.; De Souza, S.G.; Da Silva, M.L.P.; Furlan, R.; Peres, H.E.M. Study of preconcentration of non-polar compounds in microchannels with constrictions. Sens. Actuators B Chem. 2006, 115, 232–239. [Google Scholar] [CrossRef]
- Martin, M.; Crain, M.; Walsh, K.; McGill, R.A.; Houser, E.; Stepnowski, J.; Stepnowski, S.; Wu, H.-D.; Ross, S. Microfabricated vapor preconcentrator for portable ion mobility spectroscopy. Sens. Actuators B Chem. 2007, 126, 447–454. [Google Scholar] [CrossRef]
- Kanoun, O.; Müller, C.; Benchirouf, A.; Sanli, A.; Dinh, T.N.; Al-Hamry, A.; Bu, L.; Gerlach, C.; Bouhamed, A. Flexible carbon nanotube films for high performance strain sensors. Sensors 2014, 14, 10042–10071. [Google Scholar] [CrossRef] [PubMed]
- Tournus, F.; Charlier, J.C. Ab initio study of benzene adsorption on carbon nanotubes. Phys. Rev. B 2005, 71, 165421. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Hussain, C.M.; Saridara, C.; Mitra, S. Modifying the sorption properties of multi-walled carbon nanotubes via covalent functionalization. Analyst 2009, 134, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Hussain, C.M.; Saridara, C.; Mitra, S. Microtrapping characteristics of single and multi-walled carbon nanotubes. J. Chromatogr. A 2008, 1185, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hussain, C.M.; Saridara, C.; Mitra, S. Carbon nanotubes as sorbents for the gas phase preconcentration of semivolatile organics in a microtrap. Analyst 2008, 133, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/supelco/10184?lang=en®ion=US (accessed on 2 May 2018).
- Alfeeli, B.; Agah, M. Micro preconcentrator with embedded 3D pillars for breath analysis applications. In Proceedings of the IEEE Sensors Conference, Lecce, Italy, 26–29 October 2008; pp. 736–739. [Google Scholar]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lim, S.-H. CNT Foam-Embedded Micro Gas Preconcentrator for Low-Concentration Ethane Measurements. Sensors 2018, 18, 1547. https://doi.org/10.3390/s18051547
Lee J, Lim S-H. CNT Foam-Embedded Micro Gas Preconcentrator for Low-Concentration Ethane Measurements. Sensors. 2018; 18(5):1547. https://doi.org/10.3390/s18051547
Chicago/Turabian StyleLee, Janghyeon, and Si-Hyung Lim. 2018. "CNT Foam-Embedded Micro Gas Preconcentrator for Low-Concentration Ethane Measurements" Sensors 18, no. 5: 1547. https://doi.org/10.3390/s18051547