Comparison of Resonance Frequency Analysis and of Quantitative Ultrasound to Assess Dental Implant Osseointegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implants
2.2. Animal
2.3. Surgical Procedure
2.4. Experimental Protocol
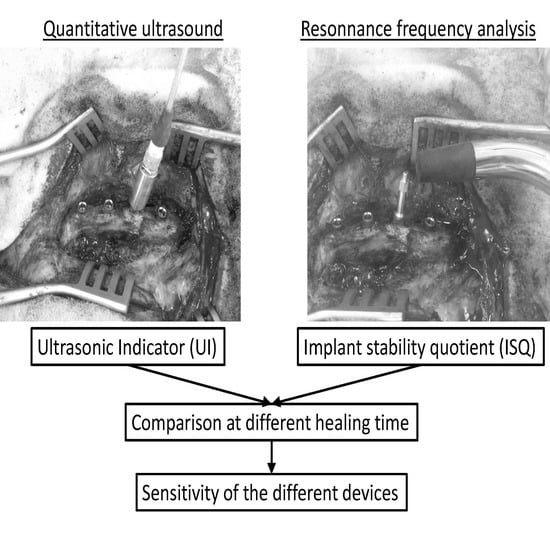
2.4.1. Quantitative Ultrasound Measurements
2.4.2. Resonnance Frequency Analysis Measurements
2.4.3. Measurement Protocol
2.4.4. Data Analysis
3. Results
3.1. Results for One Given Implant
3.2. Results Obtained When Pooling All Implants
3.3. Temporal Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Dahl, E.; Enbom, L.; Engevall, S.; Engquist, B.; Eriksson, A.R.; Feldmann, G.; Freiberg, N.; Glantz, P.O.; Kjellman, O. Osseointegrated oral implants. A swedish multicenter study of 8139 consecutively inserted nobelpharma implants. J. Periodontol. 1988, 59, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Haiat, G.; Wang, H.L.; Brunski, J.B. Effects of biomechanical properties of the bone-implant interface on dental implant stability: From in silico approaches to the patient’s mouth. Annu. Rev. Biomed. Eng. 2014, 16, 187–213. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Bacchelli, B.; Giavaresi, G.; De Pasquale, V.; Martini, D.; Fini, M.; Giardino, R.; Ruggeri, A. Influence of different implant surfaces on peri-implant osteogenesis: Histomorphometric analysis in sheep. J. Periodont. 2007, 78, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, V.; Vayron, R.; Richard, G.; Lambert, G.; Naili, S.; Meningaud, J.P.; Haiat, G. Biomechanical determinants of the stability of dental implants: Influence of the bone-implant interface properties. J. Biomech. 2014, 47, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2005, 20, 425–431. [Google Scholar]
- Buser, D.; Ingimarsson, S.; Dula, K.; Lussi, A.; Hirt, H.P.; Belser, U.C. Long-term stability of osseointegrated implants in augmented bone: A 5-year prospective study in partially edentulous patients. Int. J. Periodontics Restor. Dent. 2002, 22, 109–117. [Google Scholar]
- Serra, G.; Morais, L.S.; Elias, C.N.; Meyers, M.A.; Andrade, L.; Muller, C.; Muller, M. Sequential bone healing of immediately loaded mini-implants. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 275A, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J. The Law of Bone Remodeling; Springer: Berlin/Heidelberg, Germany, 1892. [Google Scholar]
- Hecht, S.; Adams, W.H.; Narak, J.; Thomas, W.B. Magnetic resonance imaging susceptibility artifacts due to metallic foreign bodies. Vet. Radiol. Ultrasound 2011, 52, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Bayarchimeg, D.; Namgoong, H.; Kim, B.K.; Kim, M.D.; Kim, S.; Kim, T.I.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; et al. Evaluation of the correlation between insertion torque and primary stability of dental implants using a block bone test. J. Periodontal Implant Sci. 2013, 43, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Schulte, W.; d’Hoedt, B.; Lukas, D.; Muhlbradt, L.; Scholz, F.; Bretschi, J.; Frey, D.; Gudat, H.; Konig, M.; Markl, M. Periotest—A new measurement process for periodontal function. Zahnarztl. Mitt. 1983, 73, 1229–1230. [Google Scholar] [PubMed]
- Van Scotter, D.E.; Wilson, C.J. The periotest method for determining implant success. J. Oral Implantol. 1991, 17, 410–413. [Google Scholar] [PubMed]
- Meredith, N.; Friberg, B.; Sennerby, L.; Aparicio, C. Relationship between contact time measurements and ptv values when using the periotest to measure implant stability. Int. J. Prosthodont. 1998, 11, 269–275. [Google Scholar] [PubMed]
- Valderrama, P.; Oates, T.W.; Jones, A.A.; Simpson, J.; Schoolfield, J.D.; Cochran, D. Evaluation of two different resonance frequency devices to detect implant stability: A clinical trial. J. Periodontol. 2007, 78, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implant Res. 1996, 7, 261–267. [Google Scholar] [CrossRef]
- Meredith, N.; Shagaldi, F.; Alleyne, D.; Sennerby, L.; Cawley, P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin. Oral Implant Res. 1997, 8, 234–243. [Google Scholar] [CrossRef]
- Friberg, B.; Jisander, S.; Widmark, G.; Lundgren, A.; Ivanoff, C.-J.; Sennerby, L.; Thoren, C. One-year prospective three-center study comparing the outcome of a “soft bone implant” (prototype Mk IV) and the standard branemark implant. Clin. Implant. Dent. Relat. Res. 2003, 5, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Seong, W.J.; Kim, U.K.; Swift, J.Q.; Hodges, J.S.; Ko, C.C. Correlations between physical properties of jawbone and dental implant initial stability. J. Prosthet. Dent. 2009, 101, 306–318. [Google Scholar] [CrossRef]
- Merheb, J.; Van Assche, N.; Coucke, W.; Jacobs, R.; Naert, I.; Quirynen, M. Relationship between cortical bone thickness or computerized tomography-derived bone density values and implant stability. Clin. Oral Implant. Res. 2010, 21, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, S.; Karabuda, C.; Beck, F.; Leblebicioglu, B. Resonance frequency analysis of one-stage dental implant stability during the osseointegration period. J. Periodontol. 2005, 76, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Pattijn, V.; Jaecques, S.V.N.; De Smet, E.; Muraru, L.; Van Lierde, C.; Van der Perre, G.; Naert, I.; Sloten, J.V. Resonance frequency analysis of implants in the guinea pig model: Influence of boundary conditions and orientation of the transducer. Med. Eng. Phys. 2007, 29, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Lang, N.R.; Rangert, B. Validity and clinical significance of biomechanical testing of implant/bone interface. Clin. Oral Implant. Res. 2006, 17, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Linder, E.; Lang, N.P. Implant stability in relation to osseointegration: An experimental study in the labrador dog. Clin. Oral Implant. Res. 2009, 20, 313–318. [Google Scholar] [CrossRef]
- De Almeida, M.S.; Maciel, C.D.; Pereira, J.C. Proposal for an ultrasonic tool to monitor the osseointegration of dental implants. Sensors 2007, 7, 1224–1237. [Google Scholar] [CrossRef]
- Mathieu, V.; Anagnostou, F.; Soffer, E.; Haiat, G. Ultrasonic evaluation of dental implant biomechanical stability: An in vitro study. Ultrasound Med. Biol. 2011, 37, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, V.; Vayron, R.; Soffer, E.; Anagnostou, F.; Haiat, G. Influence of healing time on the ultrasonic response of the bone-implant interface. Ultrasound Med. Biol. 2012, 38, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Karasinski, P.; Mathieu, V.; Michel, A.; Loriot, D.; Richard, G.; Lambert, G.; Haiat, G. Variation of the ultrasonic response of a dental implant embedded in tricalcium silicate-based cement under cyclic loading. J. Biomech. 2013, 46, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Mathieu, V.; Michel, A.; Haiat, G. Assessment of the in vitro dental implant primary stability using an ultrasonic method. Ultrasound Med. Biol. 2014, 40, 2885–2894. [Google Scholar]
- Vayron, R.; Soffer, E.; Anagnostou, F.; Haiat, G. Ultrasonic evaluation of dental implant osseointegration. J. Biomech. 2014, 47, 3562–3568. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Nguyen, V.; Lecuelle, B.; Haïat, G. Evaluation of dental implant stability in bone phantoms: Comparison between a quantitative ultrasound technique and resonance frequency analysis. Clin. Implant Dent. Relat. Res. 2018, in press. [Google Scholar]
- Mathieu, V.; Anagnostou, F.; Soffer, E.; Haiat, G. Numerical simulation of ultrasonic wave propagation for the evaluation of dental implant biomechanical stability. J. Acoust. Soc. Am. 2011, 129, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Nguyen, V.H.; Bosc, R.; Naili, S.; Haiat, G. Finite element simulation of ultrasonic wave propagation in a dental implant for biomechanical stability assessment. Biomech. Model. Mechan. 2015, 14, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Nguyen, V.H.; Bosc, R.; Naili, S.; Haïat, G. Assessment of dental implant biomechanical stability using quantitative ultrasound: A 3-d finite element study. J. Acoust. Soc. Am. 2016, 139, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Ravera, L.; Mortellaro, C.; Piattelli, A. Bone regeneration in iliac crestal defects: An experimental study on sheep. Biomed. Res. Int. 2016, 2016, 4086870. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. New osseodensification implant site preparation method to increase bone density in low-density bone: In vivo evaluation in sheep. Implant Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Colagiovanni, M.; Berardi, D.; Perfetti, G. Laser-treated titanium implants: An in vivo histomorphometric and biomechanical analysis. Implant Dent. 2016, 25, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of implant thread geometry on secondary stability, bone density, and bone-to-implant contact : A biomechanical and histological analysis. Implant Dent. 2015, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Kanawati, A.; Richards, M.W.; Becker, J.J.; Monaco, N.E. Measurement of clinicians’ ability to hand torque dental implant components. J. Oral Implant. 2009, 35, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Matsukawa, M.; Mathieu, V.; Barthel, E.; Soffer, E.; Anagnostou, F.; Haiat, G. Evolution of bone biomechanical properties at the micrometer scale around titanium implant as a function of healing time. Phys. Med. Biol. 2014, 59, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Salem, N.A.; Taleb, A.L.A.; Abd El Moniem, N.M. Influence of surface nano-roughness on osseointegration of zirconia implants in rabbit femur heads using selective infiltration etching technique. J. Oral Implant. 2013, 39, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ochi, M.; Abiko, Y.; Hirose, Y.; Kaku, T.; Sakaguchi, K. Pulsed electromagnetic fields promote bone formation around dental implants inserted into the femur of rabbits. Clin. Oral Implant. Res. 2000, 11, 354–360. [Google Scholar] [CrossRef]
- Kang, S.H.; Cho, J.H.; Park, S.H.; Toothaker, R.; Cho, S.A. Comparison of osseointegration between laser-etched and magnesium-incorporated oxidized implants in the rabbit femur. Int. J. Oral Maxillofac. Implant. 2013, 28, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, O.; Angelov, N.; Zafiropoulos, G.G.; Andreana, S. Osseointegration of zirconia implants with different surface characteristics: An evaluation in rabbits. Int. J. Oral Maxillofac. Implant. 2012, 27, 352–358. [Google Scholar]
- Seong, W.J.; Kim, H.C.; Jeong, S.C.; DeVeau, L.; Aparicio, C.; Li, Y.P.; Hodges, J.S. Ex vivo mechanical properties of dental implant bone cement used to rescue initially unstable dental implants: A rabbit study. Int. J. Oral Maxillofac. Implant. 2011, 26, 826–836. [Google Scholar]
- Vayron, R.; Barthel, E.; Mathieu, V.; Soffer, E.; Anagnostou, F.; Haiat, G. Nanoindentation measurements of biomechanical properties in mature and newly formed bone tissue surrounding an implant. J. Biomech. Eng. 2012, 134, 021007. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Barthel, E.; Mathieu, V.; Soffer, E.; Anagnostou, F.; Haiat, G. Variation of biomechanical properties of newly formed bone tissue determined by nanoindentation as a function of healing time. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 139–140. [Google Scholar] [CrossRef]
- Mathieu, V.; Fukui, K.; Matsukawa, M.; Kawabe, M.; Vayron, R.; Soffer, E.; Anagnostou, F.; Haiat, G. Micro-brillouin scattering measurements in mature and newly formed bone tissue surrounding an implant. J. Biomech. Eng. 2011, 133, 021006. [Google Scholar] [PubMed]
- Li, Z.H.; Muller, R.; Ruffoni, D. Bone remodeling and mechanobiology around implants: Insights from small animal imaging. J. Orthop. Res. 2018, 36, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S. Musculoskeletal Tissue Regeneration—Biological Materials and Methods; Humana Press: New York, NY, USA, 2008; p. 649. [Google Scholar]
- Liebermann, J.; Friedlaender, G. Bone Regeneration and Repair: Biology and Clinical Applications; Humana: New York, NY, USA, 2006; p. 450. [Google Scholar]






| Healing Times p–n | Indicator X | Variation | Slope aX | Temporal Sensitivity tX |
|---|---|---|---|---|
| 0–5 | UI | 31.4 | 6.3 | 0.2 |
| ISQ | 2 | 0.4 | 9.2 | |
| 5–15 | UI | −2.9 | −0.29 | 1.37 |
| ISQ | 0.67 | 0.07 | 20.6 | |
| 0–7 | UI | 27.4 | 3.9 | 0.24 |
| ISQ | 0 | 0 | ∞ |
| Healing Times p–n | 0–5 | 5–15 | 0–7 | |||
|---|---|---|---|---|---|---|
| Indicator X | UI | ISQ | UI | ISQ | UI | ISQ |
| Mean | 23.98 | −0.9 | −3.23 | −2.4 | 20.47 | 1.9 |
| Std | 14.89 | 6.6 | 15.58 | 6.3 | 18.63 | 6.9 |
| Total number of implants | 38 | 29 | 43 | |||
| Nb of implants with a significant increase of X | 36 | 5 | 11 | 10 | 37 | 8 |
| Nb of implants with a significant decrease of X | 1 | 9 | 16 | 3 | 6 | 16 |
| Nb of implants with similar values of X | 1 | 24 | 2 | 16 | 0 | 19 |
| Healing Times p–n | 0–5 | 5–15 | 0–7 | |||
|---|---|---|---|---|---|---|
| Measurement direction | 0° | 90° | 0° | 90° | 0° | 90° |
| Total number of implants | 38 | 29 | 43 | |||
| Nb of implants with a significant ISQm increase | 1 | 12 | 5 | 9 | 2 | 8 |
| Nb of implants with a significant ISQm decrease | 7 | 7 | 9 | 2 | 10 | 13 |
| Nb of implants with similar ISQm values | 30 | 19 | 15 | 18 | 31 | 22 |
| Healing Times n–p | Values | tUI | tISQ for 0° | tISQ for 90° | tISQ Pooled |
|---|---|---|---|---|---|
| 0–5 | Mean ± Std | 0.56 ± 2.04 | 4.59 ± 3.21 | 4.93 ± 4.56 | 9.58 ± 11.25 |
| Min/Max | 0.07–12.7 | 0.52–9.89 | 0.6–14.4 | 0.74–57.6 | |
| 5–15 | Mean ± Std | 1.28 ± 1.03 | 9.24 ± 11.9 | 13.93 ± 16.7 | 20.42 ± 31.8 |
| Min/Max | 0.24–3.8 | 0.97–59.31 | 1.15–86.52 | 1.27 ± 172.8 | |
| 0–7 | Mean ± Std | 0.90 ± 1.38 | 4.45 ± 5.33 | 9.67 ± 13.2 | 22.89 ± 39.4 |
| Min/Max | 0.09–6.17 | 0.33–17.64 | 0.73–75.6 | 1.29–151.2 |
| Healing Times n–p | UI | ISQ0 | ISQ90 | ISQ Both Directions Pooled |
|---|---|---|---|---|
| 0–5 | 36 | 1 | 1 | 0 |
| 5–15 | 28 | 1 | 0 | 0 |
| 0–7 | 40 | 1 | 2 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vayron, R.; Nguyen, V.-H.; Lecuelle, B.; Albini Lomami, H.; Meningaud, J.-P.; Bosc, R.; Haiat, G. Comparison of Resonance Frequency Analysis and of Quantitative Ultrasound to Assess Dental Implant Osseointegration. Sensors 2018, 18, 1397. https://doi.org/10.3390/s18051397
Vayron R, Nguyen V-H, Lecuelle B, Albini Lomami H, Meningaud J-P, Bosc R, Haiat G. Comparison of Resonance Frequency Analysis and of Quantitative Ultrasound to Assess Dental Implant Osseointegration. Sensors. 2018; 18(5):1397. https://doi.org/10.3390/s18051397
Chicago/Turabian StyleVayron, Romain, Vu-Hieu Nguyen, Benoît Lecuelle, Hugues Albini Lomami, Jean-Paul Meningaud, Romain Bosc, and Guillaume Haiat. 2018. "Comparison of Resonance Frequency Analysis and of Quantitative Ultrasound to Assess Dental Implant Osseointegration" Sensors 18, no. 5: 1397. https://doi.org/10.3390/s18051397