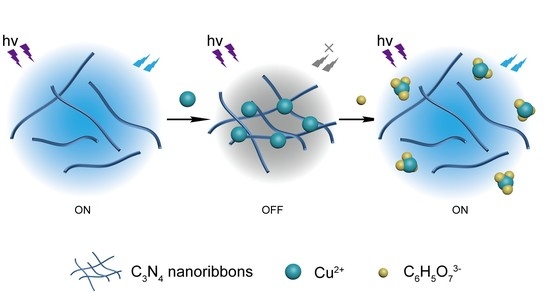
A Novel “Off-On” Fluorescent Probe Based on Carbon Nitride Nanoribbons for the Detection of Citrate Anion and Live Cell Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization
2.3. Preparation of C3N4 Nanoribbons
2.4. Synthesis of Cu2+-C3N4 Nanoribbon Complex
2.5. Fluorescent Detection of C6H5O73−
2.6. Selectivity of Cu2+-C3N4 Nanoribbon-Based Probe for C6H5O73− Detection
2.7. Cell Imaging and Cytotoxicity Assay
3. Results and Discussion
3.1. Characterization of C3N4 Nanoribbons
3.2. The Influence of Metal Ions on the Fluorescence of C3N4 Nanoribbons
3.3. Sensitivity and Selectivity of the Fluorescent “Off-On” Probe Based on C3N4 Nanoribbons for C6H5O73− Detection
3.4. Intracellular Imaging of C6H5O73−
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wise, D.R.; Ward, P.S.; Shay, J.E.S.; Cross, J.R.; Gruber, J.J.; Sachdeva, U.M.; Platt, J.M.; DeMatteo, R.G.; Simon, M.C.; Thompson, C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 2011, 108, 19611–19616. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Concepts of citrate production and secretion by prostate 1. Metab. Relatsh. Prostate 1991, 18, 25–46. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, M.J.; McDonough, K.L.; Pituch, J.J.; Christopherson, P.L.; Cornell, T.T.; Selewski, D.T.; Shanley, T.P.; Blatt, N.B. Citrate modulates lipopolysaccharide-induced monocyte inflammatory responses. Clin. Exp. Immunol. 2015, 180, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Devaraj, S.; Yang, C.; Yen, Y. A new selective chromogenic and fluorogenic sensor for citrate ion. Sens. Actuators B 2012, 174, 555–562. [Google Scholar] [CrossRef]
- Costello, L.; Franklin, R.; Narayan, P. Citrate in the diagnosis of prostate cancer. Prostate 1999, 38, 237–245. [Google Scholar] [CrossRef]
- Hikima, S.; Hasebe, K.; Taga, M. New amperometric biosensor for citrate with mercury film electrode. Electroanalysis 1992, 4, 801–803. [Google Scholar] [CrossRef]
- Peres, R.G.; Moraes, E.P.; Micke, G.A.; Tonin, F.G.; Tavares, M.F.M.; Rodriguez-Amaya, D.B. Rapid method for the determination of organic acids in wine by capillary electrophoresis with indirect UV detection. Food Control 2009, 20, 548–552. [Google Scholar] [CrossRef]
- Hasebe, K.; Hikama, S.; Yoshida, H. Determinations of citric acid by differential pulse polarography with immobilized enzymes. Fresenius J. Anal. Chem. 1990, 336, 232–234. [Google Scholar] [CrossRef]
- Miyakoshi, K.; Komoda, M. Determination of citric acid and its decomposed products in edible oils by gas liquid chromatography. J. Am. Oil Chem. Soc. 1977, 54, 331–333. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a turkish cv. Kozan. Microchem. J. 2009, 91, 187–192. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Li, Y.; Kong, X.; Zou, C.; Weng, C. Colorimetric and fluorescent chemosensor for citrate based on a rhodamine and Pb2+ complex in aqueous solution. Anal. Chim. Acta 2013, 774, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Gong, J.; Zhang, P.; Zhu, C. High-throughput and rapid fluorescent visualization sensor of urinary citrate by CdTe quantum dots. Talanta 2015, 141, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Wang, J.; Jiang, T.; Lu, N.; Hua, J. Diketopyrrolopyrrole-based ratiometric/turn-on fluorescent chemosensors for citrate detection in the near-infrared region by an aggregation-induced emission mechanism. Anal. Chem. 2016, 88, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, F.; Luo, Z.; Bao, B.; Hu, Y.; Weng, L.; Cheng, Y.; Wang, L. Conjugated polymer nanoparticles with aggregation induced emission characteristics for intracellular Fe3+ sensing. J. Polym. Sci. Part A: Polym. Chem. 2016, 54, 1686–1693. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Q.; Wu, H.; Chen, Y.; Lu, C.H.; Chi, Y.; Yang, H.H. Graphitic carbon nitride materials: Sensing, imaging and therapy. Small 2016, 12, 5376–5393. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Z.; Lee, S.U.; Heo, N.S.; Stucky, G.D.; Jun, Y.S.; Hong, W.H. A fluorescent sensor for selective detection of cyanide using mesoporous graphitic carbon(IV) nitride. Chem. Commun. 2012, 48, 3942–3944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Wang, H.; Zhang, Q.; Xie, J.; Tian, Y.; Wang, J.; Xie, Y. Single-layered graphitic-C3N4 quantum dots for two-photon fluorescence imaging of cellular nucleus. Adv. Mater. 2014, 26, 4438–4443. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ouyang, S.; Xu, H.; Wang, X.; Bi, Y.; Zhang, Y.; Ye, J. Constructing solid-gas-interfacial fnton reaction over alkalinized-C3N4 photocatalyst to achieve apparent quantum yield of 49% at 420 nm. J. Am. Chem. Soc. 2016, 138, 13289–13297. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Schnepp, Z. Soft and hard templating of graphitic carbon nitride. J. Mater. Chem. A 2015, 3, 14081–14092. [Google Scholar] [CrossRef]
- Huang, H.; Chen, R.; Ma, J.; Yan, L.; Zhao, Y.; Wang, Y.; Zhang, W.; Fan, J.; Chen, X. Graphitic carbon nitride solid nanofilms for selective and recyclable sensing of Cu2+ and Ag+ in water and serum. Chem. Commun. 2014, 50, 15415–15418. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and leds. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zeng, M.; Xu, J.; Wang, X.; Hu, W. Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 2014, 6, 4157–4162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Zheng, C.; Guo, S.S.; Li, J.; Yang, H.H.; Chen, G. Turn-on fluorescence sensor for intracellular imaging of glutathione using g-C3N4 nanosheet—MnO2 sandwich nanocomposite. Anal. Chem. 2014, 86, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X.; He, Y. Ultrathin graphitic C3N4 nanofibers: Hydrolysis-driven top-down rapid synthesis and application as a novel fluorosensor for rapid, sensitive, and selective detection of Fe3+. Sens. Actuators B 2015, 216, 453–460. [Google Scholar] [CrossRef]
- Lee, E.Z.; Jun, Y.S.; Hong, W.H.; Thomas, A.; Jin, M.M. Cubic mesoporous graphitic carbon(IV) nitride: An all-in-one chemosensor for selective optical sensing of metal ions. Angew. Chem. Int. Ed. 2010, 49, 9706–9710. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Q.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ultrathin graphitic carbon nitride nanosheet: A highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. Anal. Chem. 2013, 85, 5595–5599. [Google Scholar] [CrossRef] [PubMed]
- Field, T.B.; McCourt, J.L.; McBryde, W. Composition and stability of iron and copper citrate complexes in aqueous solution. Can. J. Chem. 1974, 52, 3119–3124. [Google Scholar] [CrossRef]
- Still, E.R.; Wikberg, P. Solution studies of systems with polynuclear complex formation. 1. The copper(II) citrate system. Inorg. Chim. Acta 1980, 46, 147–152. [Google Scholar] [CrossRef]
- Parry, R.; Dubois, F. Citrate complexes of copper in acid solutions. J. Am. Chem. Soc. 1952, 74, 3749–3753. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, X.; Li, X.; Liu, Y.; Gao, M.; Liu, X.; Kong, L.; Yang, S. Synthesis and characterization of nitrogen-rich carbon nitride nanobelts by pyrolysis of melamine. Appl. Phys. A Mater. Sci. Process. 2011, 105, 161–166. [Google Scholar] [CrossRef]
- Rong, M.; Song, X.; Zhao, T.; Yao, Q.; Wang, Y.; Chen, X. Synthesis of highly fluorescent P,O-g-C3N4 nanodots for the label-free detection of Cu2+ and acetylcholinesterase activity. J. Mater. Chem. C 2015, 3, 10916–10924. [Google Scholar] [CrossRef]
- Bai, X.; Cao, C.; Xu, X.; Yu, Q. Synthesis and characterization of crystalline carbon nitride nanowires. Solid State Commun. 2010, 150, 2148–2153. [Google Scholar] [CrossRef]
- Dementjev, A.P.; de Graaf, A.; van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon-nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Lu, X.; Gai, L.; Cui, D.; Wang, Q.; Zhao, X.; Tao, X. Synthesis and characterization of C3N4 nanowires and pseudocubic C3N4 polycrystalline nanoparticles. Mater. Lett. 2007, 61, 4255–4258. [Google Scholar] [CrossRef]
- Tang, Y.; Song, H.; Su, Y.; Lv, Y. Turn-on persistent luminescence probe based on graphitic carbon nitride for imaging detection of biothiols in biological fluids. Anal. Chem. 2013, 85, 11876–11884. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Sailatha, E.; Seshadri, S.; Kumaresan, S. FTIR, FT raman spectra and molecular structural confirmation of isoniazid. Indian J. Pure Appl. Phys. 2009, 47, 12–18. [Google Scholar]
- Gunasekaran, S.; Seshadri, S.; Muthu, S. Vibrational spectra and normal coordinate analysis of flucytosine. Indian J. Pure Appl. Phys. 2006, 44, 581–586. [Google Scholar]
- Urbaniak-Domagala, W. The use of the spectrometric technique FTIR-ATR to examine the polymers surface. In Advances Aspects Spectroscopy; Farrukh, M.A., Ed.; InTech: London, UK, 2012; pp. 85–104. ISBN 978-953-51-0715-6. [Google Scholar]
- Lin, T.; Zhong, L.; Wang, J.; Guo, L.; Wu, H.; Guo, Q.; Fu, F.; Chen, G. Graphite-like carbon nitrides as peroxidase mimetics and their applications to glucose detection. Biosens. Bioelectron. 2014, 59, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Sadhukhan, M. Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J. Mater. Chem. 2012, 22, 21832–21837. [Google Scholar] [CrossRef]
- Yang, D.; Dai, C.; Hu, Y.; Liu, S.; Weng, L.; Luo, Z.; Cheng, Y.; Wang, L. A new polymer-based fluorescent chemosensor incorporating propane-1,3-dione and 2,5-diethynylbenzene moieties for detection of copper(II) and iron(III). Polymers 2017, 9, 267. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Tan, C.; Zhang, X.; Lu, Q.; Sindoro, M.; Huang, X.; Huang, W.; Wang, L.; Zhang, H. Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications. Mater. Chem. Front. 2016, 1, 24–36. [Google Scholar] [CrossRef]
- Rajalakshmi, K.; Nam, Y.S.; Selvaraj, M.; Lee, Y.; Lee, K.B. Metal free bioimaging reagent for intracellular citrate in prostate cancer cells using aryl boronate derivative. Sens. Actuators B 2018, 259, 90–96. [Google Scholar] [CrossRef]
- Parker, D.; Yu, J. A pH-insensitive, ratiometric chemosensor for citrate using europium luminescence. Chem. Commun. 2005, 25, 3141–3143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, C.; Zhao, T.; Fu, H.W.; Wang, H.Z.; Wang, Y.J.; Kong, D.M. Photoluminescent sensing for acidic amino acids based on the disruption of graphene quantum dots/europium ions aggregates. Biosens. Bioelectron. 2015, 65, 204–210. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Yang, D.; Yang, C.; Feng, N.; Shao, Z.; Zhang, L.; Wang, X.; Weng, L.; Luo, Z.; Wang, L. A Novel “Off-On” Fluorescent Probe Based on Carbon Nitride Nanoribbons for the Detection of Citrate Anion and Live Cell Imaging. Sensors 2018, 18, 1163. https://doi.org/10.3390/s18041163
Hu Y, Yang D, Yang C, Feng N, Shao Z, Zhang L, Wang X, Weng L, Luo Z, Wang L. A Novel “Off-On” Fluorescent Probe Based on Carbon Nitride Nanoribbons for the Detection of Citrate Anion and Live Cell Imaging. Sensors. 2018; 18(4):1163. https://doi.org/10.3390/s18041163
Chicago/Turabian StyleHu, Yanling, Dongliang Yang, Chen Yang, Ning Feng, Zhouwei Shao, Lei Zhang, Xiaodong Wang, Lixing Weng, Zhimin Luo, and Lianhui Wang. 2018. "A Novel “Off-On” Fluorescent Probe Based on Carbon Nitride Nanoribbons for the Detection of Citrate Anion and Live Cell Imaging" Sensors 18, no. 4: 1163. https://doi.org/10.3390/s18041163