Genotyping and Bio-Sensing Chemosensory Proteins in Insects
Abstract
:1. Introduction
2. Intron Variation and Retrotransposition in a Conserved Gene Family
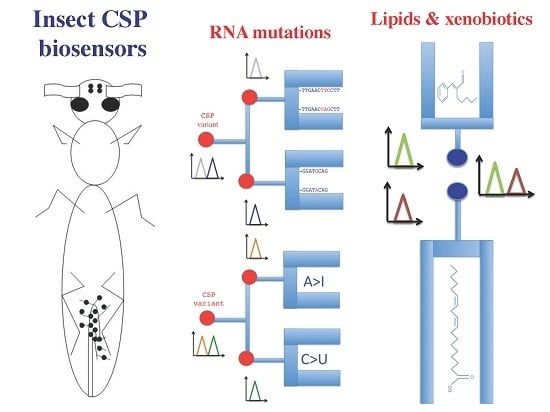
3. Biosensing Expression of Tissue-Specific RNA Mutations
4. Protein Structures for Multi-Lipid and Multi-Insecticide Sensors
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Picimbon, J.F. Olfactory Concepts of Insect Control; Springer: Dordrecht, The Netherlands, 2017; in press. [Google Scholar]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Frohlich, D.R.; Rosell, R.C. The sweet potato or silverleaf whiteflies: Biotype of Bemisia tabaci or a species complex. Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 2005, 58, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, M.; Kontsedalov, S. Gene expression in pyriproxyfen-resistant Bemisia tabaci Q biotype. Pest Manag. Sci. 2007, 63, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insecticide resistance to neonicotinoid insecticides. Pest. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.L.; Zheng, Y.; Huang, X.Y.; Ding, X.L.; Zhao, J.W.; Desneux, N.; He, Y.X. Dynamics of Bemisia tabaci biotypes and insecticide resistance in Fujian province in China during 2005–2014. Sci. Rep. 2017, 7, 40803. [Google Scholar] [CrossRef] [PubMed]
- Wool, D.; Noiman, S.; Manheim, O.; Cohen, E. Malathion resistance in Tribolium strains and their hybrids: Inheritance patterns and possible enzymatic mechanisms (Coleoptera, Tenebrionidae). Biochem. Genet. 1982, 20, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.; Kreitman, M.; Phillips, T.W.; Beeman, R.W.; ffrench-Constant, R.H. Multiple origins of cyclodiene insecticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae). J. Mol. Evol. 1999, 48, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Sadd, B.M.; Schmid-Hempel, P.; Kurtz, J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. R. Soc. B 2009, 276, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Parthasarathy, R.; Ba, H.; Woithe, K.; Kaussmann, M.; Nauen, R.; Harrison, D.A.; Palli, S.R. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, L.; Brostaux, Y.; Assié, L.K.; Gaspar, C.; Haubruge, E. Increased fecundity of malathion-specific resistant beetles in absence of insecticide pressure. Heredity 2002, 89, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Bindroo, B.B.; Moorthy, S.M. Genetic divergence, implication of diversity, and conservation of silkworm, Bombyx mori. Int. J. Biodiv. 2014. [Google Scholar] [CrossRef]
- Liu, G.X.; Xuan, N.; Chu, D.; Xie, H.Y.; Fan, Z.X.; Bi, Y.P.; Picimbon, J.F.; Qin, Y.C.; Zhong, S.T.; Li, Y.F.; et al. Biotype expression and insecticide response of Bemisia tabaci chemosensory protein-1. Arch. Insect Biochem. Physiol. 2014, 85, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Ma, H.M.; Xie, H.Y.; Xuan, N.; Picimbon, J.F. Sequence variation of Bemisia tabaci chemosensory protein 2 in cryptic species B and Q: New DNA markers for whitefly recognition. Gene 2016, 576, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Ma, H.M.; Xie, Y.N.; Xuan, N.; Xia, G.; Fan, Z.X.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.F. Biotype characterization, developmental profiling, insecticide response and binding property of Bemisia tabaci chemosensory proteins: Role of CSP in insect defense. PLoS ONE 2016, 11, e0154706. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.C.; Chen, C.J.; Li, W.H.; Chuang, T.J. Gene family size conservation is a good indicator of evolutionary rates. Mol. Biol. Evol. 2010, 27, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, P.H.; Gravemeyer, J.; Rauscher, M.; Wiehe, T. Ultra large gene families: A matter of adaptation or genomic parasites? Life 2016, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.R.; Han, K.Y.; Jung, J.; Kim, S.K. Qu Quantitative genotyping of single nucleotide polymorphism by single-molecule multi-color fluorescence resonance energy transfer. Chem. Commun. 2011, 7, 10362–10364. [Google Scholar] [CrossRef] [PubMed]
- Malkki, M.; Petersdorf, E.W. Genotyping of single nucleotide polymorphisms by 5′ nuclease allelic discrimination. Methods Mol. Biol. 2012, 882. [Google Scholar] [CrossRef]
- Vaisman, B.L. Genotyping of transgenic animals by real-time quantitative PCR with TaqMan probes. Methods Mol. Biol. 2013, 1027, 233–251. [Google Scholar] [PubMed]
- Lee, H.B.; Schwab, T.L.; Koleilat, A.; Ata, H.; Daby, C.L.; Lopez Cervera, R.; McNulty, M.S.; Bostwick, H.S.; Clark, K.J. Allele-specific quantitative PCR for accurate, rapid, and cost-effective genotyping. Hum. Gene Ther. 2016, 27, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, F.; Lv, B.; Xhao, P.; Ma, X. Suspension Array for Multiplex Detection of Eight Fungicide-Resistance Related Alleles in Botrytis cinerea. Front. Microbiol. 2016, 7, 1482. [Google Scholar] [CrossRef] [PubMed]
- Ka, S.; Lee, S.; Hong, J.; Cho, Y.; Sung, J.; Kim, H.N.; Kim, H.L.; Jung, J. HLAscan: Genotyping of the HLA region using next-generation sequencing data. BMC Bioinform. 2017, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Picimbon, J.F. Biochemistry and evolution of CSP and OBP proteins. In Insect Pheromone Biochemistry and Molecular Biology—The Biosynthesis and Detection of Pheromones and Plant Volatiles; Blomquist, G.J., Vogt, R.G., Eds.; Elsevier Academic Press: London, UK, 2003; pp. 539–566. [Google Scholar]
- Picimbon, J.F.; Dietrich, K.; Krieger, J.; Breer, H. Identity and expression pattern of chemosensory proteins in Heliothis virescens (Lepidoptera, Noctuidae). Insect Biochem. Mol. Biol. 2001, 31, 1173–1181. [Google Scholar] [CrossRef]
- Sabatier, L.; Jouanguy, E.; Dostert, C.; Zachary, D.; Dimarcq, J.L.; Bulet, P.; Imler, J.L. Pherokine-2 and -3: Two Drosophila molecules related to pheromone/odor-binding proteins induced by viral and bacterial infections. Eur. J. Biol. 2003, 270, 3398–3407. [Google Scholar] [CrossRef]
- Maleszka, J.; Forêt, S.; Saint, R.; Maleszka, R. RNAi-induced phenotypes suggest a novel role for a chemosensory protein CSP5 in the development of embryonic integument in the honeybee (Apis mellifera). Dev. Genes Evol. 2007, 217, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Xuan, N.; Guo, X.; Xie, H.Y.; Lou, Q.N.; Bo, L.X.; Liu, G.X.; Picimbon, J.F. Increased expression of CSP and CYP genes in adult silkworm females exposed to avermectins. Insect Sci. 2015, 22, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Picimbon, J.F. Bacterial origin of chemosensory odor-binding proteins. Gene Transl. Bioinform. 2017, 3, e1548. [Google Scholar]
- Zhang, Y.; Stommel, J.R. Development of SCAR and CAPS markers linked to the βeta gene in tomato. Crop Sci. 2001, 41, 1602–1608. [Google Scholar] [CrossRef]
- Yang, L.; Fu, S.; Khan, M.A.; Zeng, W.; Fu, J. Molecular cloning and development of RAPD-SCAR markers for Dimocarpus longan variety authentication. SpringerPlus 2013, 2, 501. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Leterme, N.; Latorre, A. Molecular markers linked to breeding system differences in segregating and natural populations of the cereal aphid Rhopalosiphum padi L. Mol. Ecol. 1999, 8, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Agusti, N.; De Vicente, M.C.; Gabarra, R. Development of sequence amplified characterized region (SCAR) markers of Helicoverpa armigera: A new polymerase chain reaction-based technique for predator gut analysis. Mol. Ecol. 2000, 8, 1467–1474. [Google Scholar] [CrossRef]
- Manguin, S.; Kengne, P.; Sonnier, L.; Harbach, R.E.; Baimai, V.; Trung, H.D.; Coosemans, M. SCAR markers and multiplex PCR-based identification of isomorphic species in the Anopheles dirus complex in Southeast Asia. Med. Vet. Entomol. 2002, 16, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kethidi, D.R.; Roden, D.B.; Ladd, T.R.; Krell, P.J.; Retnakaran, A.; Feng, Q. Development of SCAR markers for the DNA-based detection of the Asian long-horned beetle, Anoplophora glabripennis (Motschulsky). Arch. Insect Biochem. Physiol. 2003, 52, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, P.R.; Martins, E.S.; Klautau, N.; Lima, L.; Praça, L.; Monnerat, R.G. Identification of the B, Q, and native Brazilian biotypes of the Bemisia tabaci species complex using Scar markers. Pesq. Agropec. Bras. 2016, 51, 555–562. [Google Scholar] [CrossRef]
- Rauch, N.; Nauen, R. Identification of biochemical markers linked to neonicotinoid cross resistance in Bemisia tabaci (Hemiptera: Aleyrodidae). Arch. Insect Biochem. Physiol. 2003, 54, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Li, Y.; Bai, X.; Cai, H.; Ji, Z.; Guo, C.; Zhu, Y. Identification and characterization of a new member of the SINE Au retroposon (GmAu1) in the soybean, Glycine max (L.) Merr., genome and its potential application. Plant Cell Rep. 2011, 30, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Izsvák, Z.; Ivics, Z.; Hackett, P.B. Repetitive elements and their genetic applications in zebrafish. Biochem. Cell Biol. 1997, 75, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Behura, S.K. Molecular marker systems in insects: current trends and future avenues. Mol. Ecol. 2006, 15, 3087–3113. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Cavalcani, A.R. Consequences of stop codon reassignment on protein evolution in ciliates with alternative genetic codes. Mol. Biol. Evol. 2008, 25, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Vakhrusheva, A.A.; Kazanov, M.D.; Mironov, A.A.; Bazykin, G.A. Evolution of prokaryotic genes by shift of stop codons. J. Mol. Evol. 2011, 72, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lynch, M. Ubiquitous internal gene duplication and intron creation in eukaryotes. Proc. Natl. Acad. Sci. USA 2009, 106, 20818–20823. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.T.; Zilberman, D.; Roy, S.W. Mechanism for DNA transposons to generate introns on genomic scales. Nature 2016, 538, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Tribolium Genome Sequencing Consortium. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar]
- Van de Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Marín, M.; Buggs, R.J.A.; Cooley, A.M.; Puzey, J.R. Speciation by genome duplication: Repeated origins and genomic composition of the recently formed allopolyploid species Mimulus peregrinus. Evolution 2015, 69, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duhl, D.M.; Barsh, G.S. Opposite orientations of an inverted duplication and allelic variation at the mouse agouti locus. Genetics 1996, 144, 265–277. [Google Scholar] [PubMed]
- Babenko, V.N.; Rogozin, I.B.; Mekhedov, S.L.; Koonin, E.V. Prevalence of intron gain gain over intron loss in the evolution of paralogous gene families. Nucl. Acids Res. 2004, 32, 3724–3733. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Lapoint, R.T.; Whiteman, N.K. Herbivory increases diversification across insect clades. Nat. Commun. 2015, 6, 8370. [Google Scholar] [CrossRef] [PubMed]
- Yenerall, P.; Krupa, B.; Zhou, L. Mechansisms of intron gain and loss in Drosophila. BMC Evol. Biol. 2011, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Beeman, R.W.; Thomson, M.S.; Clark, J.M.; DeCamillis, M.A.; Brown, S.J.; Denell, R.E. Woot, an active gypsy-class retrotransposon in the flour beetle, Tribolium castaneum, is associated with a recent mutation. Genetics 1996, 143, 417–426. [Google Scholar] [PubMed]
- Goodall, G.J.; Filipowicz, W. The minimum functional length of pre-mRNA introns in monocots and dicots. Plant Mol. Biol. 1990, 14, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Mount, S.C.; Burks, C.; Hertz, G.; Stormo, G.D.; White, O.; Fields, C. Splicing signals in Drosophila: Intron size, information content, and consensus sequences. Nucl. Acids Res. 1992, 20, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Haraguchi, N.; Shimada, M.K.; Taniguchi, I.; Ohno, M.; Mayeda, A. Mechanistic insights into human pre-mRNA splicing of human ultra-short introns: Potential unusual mechanism identifies G-rich introns. Biochem. Biophys. Res. Commun. 2012, 423, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Xuan, N.; Bu, X.; Liu, Y.Y.; Yang, X.; Liu, G.X.; Fan, Z.X.; Bi, Y.P.; Yang, L.Q.; Lou, Q.N.; Rajashekar, B.; et al. Molecular evidence of RNA editing in Bombyx chemosensory protein family. PLoS ONE 2014, 9, e86932. [Google Scholar] [CrossRef] [PubMed]
- Xuan, N.; Rajashekar, B.; Kasvandik, S.; Picimbon, J.F. Structural components of chemosensory protein mutations in the silkworm moth, Bombyx mori. Agric. Gene 2016, 2, 53–58. [Google Scholar] [CrossRef]
- Picimbon, J.F. RNA mutations in the moth pheromone gland. RNA Dis. 2014, 1, e240. [Google Scholar]
- Picimbon, J.F. RNA mutations: Source of life. Gene Technol. 2014, 3, 112–122. [Google Scholar] [CrossRef]
- Picimbon, J.F. Mutations in the insect transcriptome. J. Clin. Exp. Pathol. 2016, 6, 3. [Google Scholar]
- Picimbon, J.F. A new view of genetic mutations. Australas. Med. J. 2017, in press. [Google Scholar]
- Picimbon, J.F. Evolution of protein physical structures in insect chemosensory systems. In Olfactory Concepts of Insect Control; Picimbon, J.F., Ed.; Springer-SBM: Dordrecht, The Netherlands, 2017; in press. [Google Scholar]
- Lorenzen, M.D.; Doyungan, Z.; Savard, J.; Snow, K.; Crumly, L.R.; Shippy, T.D.; Stuart, J.J.; Brown, S.J.; Beeman, R.W. Genetic linkage maps of the red flour beetle, Tribolium castaneum, based on bacterial artificial chromosomes and expressed sequence tags. Genetics 2005, 170, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Aikins, J.A.; Wang, L.J.; Beeman, R.W.; Oppert, B.; Lord, J.C.; Brown, S.J.; Lorenzen, M.D.; Richards, S.; Weidstock, G.M.; et al. Analysis of transcriptome data in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2007, 38, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Qi, J.; Zhou, X.F.; Hu, M.Y.; Zhong, G.H. Differential expression of chemosensory-protein genes in midguts in response to diet of Spodoptera litura. Sci. Rep. 2017, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Angeli, S.; Ceron, F.; Scaloni, A.; Monti, M.; Monteforti, G.; Minnocci, A.; Petacchi, R.; Pelosi, P. Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria. Eur. J. Biochem. 1999, 262, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Picimbon, J.F. Renaming Bombyx mori Chemosensory Proteins. Int. J. Bioorg. Chem. Mol. Biol. 2014, 2, 201–204. [Google Scholar]
- McGettigan, J.; McLennan, R.K.J.; Broderick, K.E.; Kean, L.; Allan, A.K.; Cabrero, P.; Regulski, M.R.; Pollock, V.P.; Gould, G.W.; Davies, S.A.; et al. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem. Mol. Biol. 2005, 35, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Yang, B.; Xu, Q.; Li, X.; Lu, Z.; Wang, C.; Huang, Y.; Söderhäll, K.; Ling, E. Hindgut innate immunity and regulation of fecal microbiota through melanization in insects. J. Biol. Chem. 2012, 287, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Liu, L.Y.; Tsai, W.H.; Haymer, D.S.; Lu, K.H. Using DNA chips for identification of tephritid pest species. Pest Manag. Sci. 2014, 70, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.R.; Gloria-Soria, A.; Hou, L.; McBride, C.; Bonizzoni, M.; Zhao, H.; Powell, J.R. A multipurpose, high-throughput single-nucleotide polymorphism chip for the dengue and yellow fever mosquito, Aedes aegypti. G3 2015, 5, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Coe, B.P.; Eichler, E.E. Genome structural variation discovery and genotyping. Nat. Rev. Genet. 2011, 12, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Berber, E.; Leggo, J.; Brown, C.; Berber, E.; Gallo, N.; Feilotter, H.; Lillicrap, D. DNA microarray analysis for the detection of mutations in hemophilia A. J. Thromb. Haemost. 2006, 4, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Lichtenstein, A.; Willner, I. Detection of single-base DNA mutations by enzyme-amplified electronic transduction. Nat. Biotech. 2001, 19, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nat. Biotech. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S.; Rovina, K.; Yusof, N.A.; Rodrigues, K.F.; Suryani, S. Nanoparticle-enhanced electrochemical biosensor with DNA immobilization and hybridization of Trichoderma harzianum gene. Sens. Biosens. Res. 2014, 2, 16–22. [Google Scholar] [CrossRef]
- Rahman, M.M.; Li, X.B.; Lopa, N.S.; Ahn, S.J.; Lee, J.J. Electrochemical DNA hybridization sensors based on conducting polymers. Sensors 2015, 15, 3801–3829. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Kitaoka, M.; Okamura, N.; Maruyama, T.; Kamiya, N.; Goto, M. Detection of single-base mutations by fluorogenic ribonuclease protection assay. Anal. Chem. 2005, 77, 7047–7053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.E.; Bi, L.J. Protein chip for detection of DNA mutations. Methods Mol. Biol. 2007, 382, 163–176. [Google Scholar] [PubMed]
- Hotting, J.; Morean, J.; Roger, G.; Spadavecchia, J.; Millot, M.C.; Goossens, M.; Canva, M. Plasmonic DNA: Towards genetic diagnosis chips. Plasmonics 2007, 2, 201–215. [Google Scholar] [CrossRef]
- Duan, R.Q.; Yuan, J.L.; Yang, H.; Luo, X.G.; Xi, M.R. Detection of p53 gene mutation by using a novel biosensor based on localized surface plasmon resonance. Neoplasma 2012, 59, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Barricarte, R.; Heurich, M.; López-Perrote, A.; Tortajada, A.; Pinto, S.; López-Trascasa, M.; Sánchez-Corral, P.; Morgan, P.; Llorca, O. The molecular and structural bases for the association of complement C3 mutations with atypical hemolytic uremic symdrome. Mol. Immunol. 2015, 66, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.L.; Weintraub, H. A developmentally regulated activity that unwinds RNA duplexes. Cell 1987, 48, 607–613. [Google Scholar] [CrossRef]
- Chester, A.; Scott, J.; Anant, S.; Navaratnam, N. RNA editing: Cytidine to uridine conversion in apolipoprotein B mRNA. Biochim. Biophys. Acta 2000, 1494, 1–13. [Google Scholar] [CrossRef]
- Hundley, H.A.; Bass, B.L. ADAR editing in double-stranded UTRs and other noncoding RNA sequences. Trends Biochem. Sci. 2010, 35, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010, 79, 321–349. [Google Scholar] [CrossRef] [PubMed]
- Maas, S. Gene regulation through RNA editing. Discov. Med. 2010, 10, 379–386. [Google Scholar] [PubMed]
- Maas, S.; Rich, A. Changing genetic information through RNA editing. BioEssays 2000, 22, 790–802. [Google Scholar] [CrossRef]
- Dawson, T.R.; Sansam, C.L.; Emeson, R.B. Structure and sequence determinants required for the RNA editing of ADAR2 substrates. J. Biol. Chem. 2004, 279, 4941–4951. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, C.; Bransteitter, R.; Klein, M.G.; Goodman, M.F.; Chen, X.S. The APOBEC-2 crystal structure and functional implications for the deaminase AID. Nature 2007, 445, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.A.; Pastar, I.; Gaston, K.W.; Ragone, F.L.; Janzen, C.J.; Cross, G.A.M.; Papavasiliou, F.N.; Alfonzo, J.D. An adenosine-to-inosine tRNA-editing enzyme that can perform C-to-U deamination of DNA. Proc. Natl. Acad. Sci. USA 2007, 104, 7821–7826. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.A.; Macbeth, M.R.; Beal, P.A. ADAR proteins: Structure and catalytic mechanism. Curr. Top. Microbiol. Immunol. 2012, 353, 1–33. [Google Scholar] [PubMed]
- Thomas, J.M.; Beal, P.A. How do ADARs bind RNA? New protein-RNA structures illuminate substrate recognition by the RNA editing ADARs. BioEssays 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Tajaddod, M.; Jantsch, F.; Licht, K. The dynamic epitranscriptome: A to I editing modulates genetic information. Chromosoma 2016, 125, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Deng, P.; Jacobson, D.; Li, J.B. Evolutionary analysis reveals regulatory and functional landscape of coding and non-coding RNA editing. PLoS Genet. 2017, 13, e1006563. [Google Scholar] [CrossRef] [PubMed]
- Randau, L.; Stanley, B.J.; Kohlway, A.; Mechta, S.; Xiong, Y.; Söll, D. A cytidine deaminase edits C to U in transfer RNAs in Archaea. Science 2009, 324, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Nainar, S.; Marshall, P.R.; Tyler, C.R.; Spitale, R.C.; Bredy, T.W. Evolving insights into RNA modifications and their functional diversity in the brain. Nat. Neurosci. 2016, 19, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.A.T.; Gaston, K.W.; McKenney, K.M.; Fleming, I.M.C.; Paris, Z.; Limbach, P.A.; Alfonzo, J.D. Editing and methylation at a single site by functionally interdependent activities. Nature 2017, 542, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, A.; Campanacci, V.; Roussel, A.; Larsson, A.M.; Jones, T.A.; Tegoni, M.; Cambillau, C. X-ray structure and ligand binding study of a moth chemosensory protein. J. Biol. Chem. 2002, 277, 32094–32098. [Google Scholar] [CrossRef] [PubMed]
- Briand, L.; Swasdipan, N.; Nespoulos, C.; Bézirard, V.; Blon, F.; Huet, J.C.; Ebert, J.C.; Pernollet, J.C. Characterization of a chemosensory protein (ASP3c) from honeybee (Apis mellifera L.) as a brood pheromone carrier. Eur. J. Biochem. 2002, 269, 4586–4596. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, A.; Campanacci, V.; Lartigue, A.; Tegoni, M.; Cambillau, C.; Darbon, H. Solution structure of a chemosensory protein from the moth Mamestra brassicae. Biochem. J. 2003, 369, 39–44. [Google Scholar] [CrossRef] [PubMed]
- González, D.; Zhao, Q.; McMahan, C.; Velasquez, D.; Haskins, W.E.; Sponsel, V.; Cassill, A.; Renthal, R. The major antenna; chemosensory protein of red imported fire and workers. Insect Mol. Biol. 2009, 18, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, S.; Liou, Y.C.; Davies, P.L.; Krause, E.; Sönnichsen, F.D. A new class of hexahelical insect proteins revealed as putative carriers of small hydrophobic ligands. Structure 1999, 7, 1325–1332. [Google Scholar] [CrossRef]
- Ishida, Y.; Tsuchiya, W.; Fujii, T.; Fujimoto, Z.; Miyazawa, M.; Ishibashi, J.; Matsuyama, S.; Ishikawa, Y.; Yamazaki, T. Niemann-Pick type C2 protein mediating chemical communication in the worker ant. Proc. Natl. Acad. Sci. USA 2014, 111, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Picimbon, J.F.; Dietrich, K.; Breer, H.; Krieger, J. Chemosensory proteins of Locusta migratoria (Orthoptera: Acrididae). Insect Biochem. Mol. Biol. 2000, 30, 233–241. [Google Scholar] [CrossRef]
- Ozaki, M.; Wada-Katsumata, A.; Fujikawa, K.; Iwasaki, M.; Yokohari, F.; Satoji, Y.; Nisimura, T.; Yamaoka, R. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 2005, 309, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F. Cuticular lipids as a cross-talk among ants, plants and butterflies. Int. J. Mol. Sci. 2016, 17, 1966. [Google Scholar] [CrossRef] [PubMed]
- Daikoku, T.; Yano, I.; Masul, M. Lipid and fatty acid compositions and their changes in the different organs and tissues of guppy, poecilia reticulate on sea water adaptation. Comp. Biochem. Physiol. Part A 1982, 73, 167–174. [Google Scholar] [CrossRef]
- Laino, A.; Mattoni, C.; Ojanguren-Affilastro, A.; Cunningham, M.; Garcia, F. Analysis of lipid and fatty acid composition of three species of scorpions with relation to different organs. Comp. Biochem. Physiol. B 2015, 190, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vézilier, J.; Nicot, A.; Gandon, S.; Rivero, A. Insecticide resistance and malaria transmission: Infection rate and oocyst burden in Culex pipiens infected with Plasmodium relictum. Malar. J. 2010, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.D.; Leger, E.; Dietrich, M. Host specialization in ticks and transmission of tick-borne diseases: A review. Front. Cell. Infect. Microbiol. 2013, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Liu, X.L.; Fu, J. Lactobacillus tritherapy for cholesterol and heart diseases. RR J. Microbiol. Biotechnol. 2016, 5, 23–26. [Google Scholar]
- Lungarini, S.; Aureli, F.; Coni, E. Coumarin and cinnamaldehyde in cinnamon marked in Italy: A natural chemical hazard. Food Addit. Contam. Part A 2008, 25, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Avula, B.; Nanayakkara, N.P.D.; Zhao, J.; Khan, I.A. Cassia cinnamon as a source of Coumarin in Cinnamon-flavored food and food supplements in the United States. J. Agric. Food Chem. 2013, 61, 4470. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, F.; Zhao, L.; Tan, J.; Jiang, H.; Hu, F. Neonicotinoid insecticide interact with honeybee odorant-binding protein: Implication for olfactory dysfunction. Int. J. Biol. Macromol. 2015, 81, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Han, G.; Du, Y.; Wang, J. Lack of cross-resistance between neonicotinoids and sulfoxaflor in field strains of Q-biotype of whitefly, Bemisia tabaci, from Eastern China. Pest. Biochem. Physiol. 2017, 136, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Persaud, K.C.; Tuccori, E. Biosensors based on Odorant Binding Proteins. In Bioelectronic Nose—Integration of Biotechnology and Nanotechnology; Park, T.H., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 171–190. [Google Scholar]
- Larisika, M.; Kotlowski, C.; Steininger, C.; Mastrogiacomo, R.; Pelosi, P.; Schütz, S.; Peteu, S.F.; Kleber, C.; Reiner-Rozman, C.; Nowak, C.; et al. Electronic olfactory sensor based on A. mellifera odorant-binding protein 14 on a reduced grapheme oxide field-effect transistor. Angew. Chem. Int. Ed. Engl. 2015, 54, 13245–13248. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Arnaud, P.; Offmann, B.; Picimbon, J.-F. Genotyping and Bio-Sensing Chemosensory Proteins in Insects. Sensors 2017, 17, 1801. https://doi.org/10.3390/s17081801
Liu G, Arnaud P, Offmann B, Picimbon J-F. Genotyping and Bio-Sensing Chemosensory Proteins in Insects. Sensors. 2017; 17(8):1801. https://doi.org/10.3390/s17081801
Chicago/Turabian StyleLiu, Guoxia, Philippe Arnaud, Bernard Offmann, and Jean-François Picimbon. 2017. "Genotyping and Bio-Sensing Chemosensory Proteins in Insects" Sensors 17, no. 8: 1801. https://doi.org/10.3390/s17081801