Fine-Scale Mapping of Natural Ecological Communities Using Machine Learning Approaches
Abstract
:1. Introduction
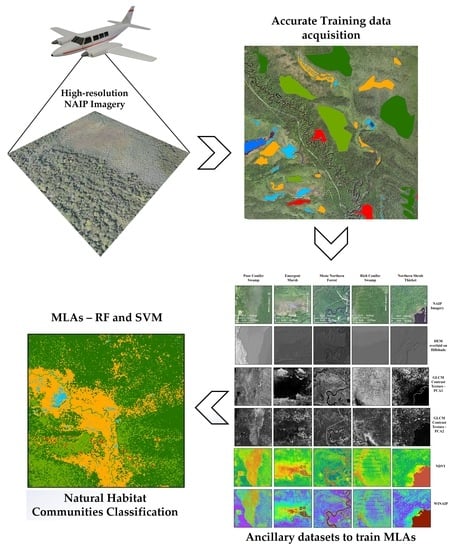
2. Study Area, Data and Methods
2.1. Study Area
2.2. Imagery
2.3. Methods
2.3.1. Utility of Image Transformations
2.3.2. Vegetation and Moisture Indices
2.3.3. Texture
2.3.4. Topographical Characteristics
2.3.5. Selection of Input Ancillary Data Layers
- Four bands of NAIP imagery;
- Elevation from DEM;
- Contrast texture (C1*7) calculated from PC1 (7 × 7) moving window, C2*7 calculated from PC2 (7 × 7) moving window;
- Normalized vegetation index (NDVI);
- Modified water index (WINAIP) derived from NAIP imagery.
2.3.6. Collection of Training Samples
2.3.7. Utilization of MLAs for Natural Communities Classification
2.3.8. Random Forest
2.3.9. Support Vector Machine
2.3.10. Accuracy Assessment and Classification Differences
2.3.11. Classification Post-Processing
3. Results
3.1. Ancillary Data and Feature Selection Methods
3.2. Classifications Results
4. Discussion
4.1. Feature Selection and Imprtance of Ancillary Datasets
4.2. MLAs Classifier Performance
4.3. Overall Performance of MLAs for Natural Habitat Communities Classification
4.4. Importance of Reference Vegetation Map
4.5. Impact of Number of Training Samples and Quality of Sample Data on MLAs
4.6. Validation of Classified Community Vegetation Map Using Field Data and Expert Observation
4.7. Future Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lincoln, R.J.; Boxshall, G.; Clark, P.F. A Dictionary of Ecology, Evolution and Systematics. Syst. Bot. 1983, 8, 339. [Google Scholar] [CrossRef]
- Tansley, A.G. The Use and Abuse of Vegetational Concepts and Terms. Ecology 1935, 16, 284–307. [Google Scholar] [CrossRef]
- Bailey, R.G. Ecosystem Geography: From Ecoregions to Sites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bailey, R.G. Identifying ecoregion boundaries. Environ. Manag. 2004, 34, S14–S26. [Google Scholar] [CrossRef] [PubMed]
- Loucks, O.L. A forest classification for the Maritime Provinces. Proc. Nova Scotian Inst. Sci. 1962, 25, 1958–1962. [Google Scholar]
- Bailey, R.G. Delineation of ecosystem regions. Environ. Manag. 1983, 7, 365–373. [Google Scholar] [CrossRef] [Green Version]
- King, A.W. Considerations of Scale and Hierarchy. In Ecological Integrity and the Management of Ecosystems; CRC Press: Boca Raton, FL, USA, 2020; pp. 19–45. [Google Scholar] [CrossRef]
- Bailey, R. Ecoregions of the United States (Map); US Department of Agriculture, US Forest Service, Intermountain Region: Ogden, UT, USA, 1976. [Google Scholar]
- Barnes, B.V. The landscape ecosystem approach and conservation of endangered spaces. Endanger. Species Update 1993, 10, 13–19. [Google Scholar]
- Rowe, J.S.; Barnes, B.V. Geo-ecosystems and bio-ecosystems. Bull. Ecol. Soc. Am. 1994, 75, 40–41. [Google Scholar]
- Cowardin, L.M.; Carter, V.; Golet, F.C.; LaRoe, E.T. Classification of Wetlands and Deepwater Habitats of the United States; US Department of the Interior, US Fish and Wildlife Service: Washington, DC, USA, 1979. [Google Scholar]
- Cohen, J.G.; Kost, M.A.; Slaughter, B.S.; Albert, D.A. A Field Guide to the Natural Communities of Michigan; Michigan State University Press: East Lansing, MI, USA, 2014. [Google Scholar]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices, 3rd ed.; Chapman and Hall/CRC: Milton, ON, Canada, 2019. [Google Scholar]
- Bergen, K.M.; Dronova, I. Observing succession on aspen-dominated landscapes using a remote sensing-ecosystem approach. Landsc. Ecol. 2007, 22, 1395–1410. [Google Scholar] [CrossRef]
- Chapman, K.A. Michigan Natural Community Types; Michigan Natural Features Inventory, Ohio Dept of Natural Resources: Columbus, OH, USA, 1986. [Google Scholar]
- Kost, M.; Cohen, J.; Albert, D.A.; Slaughter, B.; Schillo, R.K.; Weber, C.R.; Chapman, K. Natural Communities of Michigan: Classification and Description; Michigan Natural Features Inventory: Lansing, MI, USA, 2007. [Google Scholar] [CrossRef]
- Homer, C.; Huang, C.; Yang, L.; Wylie, B.; Coan, M. Development of a 2001 National Land-Cover Database for the United States. Photogramm. Eng. Remote Sens. 2004, 70, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Lunetta, R. Wetland and Environmental Application of GIS; Lewis Publishers: New York, NY, USA, 1996. [Google Scholar]
- Silva, T.S.F.; Costa, M.P.F.; Melack, J.M.; Novo, E.M.L.M. Remote sensing of aquatic vegetation: Theory and applications. Environ. Monit. Assess. 2007, 140, 131–145. [Google Scholar] [CrossRef]
- Ozesmi, S.L.; Bauer, M.E. Satellite remote sensing of wetlands. Wetl. Ecol. Manag. 2002, 10, 381–402. [Google Scholar] [CrossRef]
- Rundquist, D.C.; Narumalani, S.; Narayanan, R.M. A review of wetlands remote sensing and defining new considerations. Remote Sens. Rev. 2001, 20, 207–226. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Olmo, M.C.; Abarca-Hernandez, F.; Atkinson, P.; Jeganathan, C. Random Forest classification of Mediterranean land cover using multi-seasonal imagery and multi-seasonal texture. Remote Sens. Environ. 2012, 121, 93–107. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Ghimire, B.; Rogan, J.; Olmo, M.C.; Rigol-Sanchez, J.P. An assessment of the effectiveness of a random forest classifier for land-cover classification. ISPRS J. Photogramm. Remote Sens. 2012, 67, 93–104. [Google Scholar] [CrossRef]
- Yang, L.; Jin, S.; Danielson, P.; Homer, C.; Gass, L.; Bender, S.M.; Case, A.; Costello, C.; Dewitz, J.; Fry, J.; et al. A new generation of the United States National Land Cover Database: Requirements, research priorities, design, and implementation strategies. ISPRS J. Photogramm. Remote Sens. 2018, 146, 108–123. [Google Scholar] [CrossRef]
- Latifovic, R.; Zhu, Z.-L.; Cihlar, J.; Giri, C.; Olthof, I. Land cover mapping of North and Central America—Global Land Cover 2000. Remote. Sens. Environ. 2004, 89, 116–127. [Google Scholar] [CrossRef]
- Hansen, M.C.; DeFries, R.S.; Townshend, J.R.G.; Sohlberg, R.A. Global land cover classification at 1 km spatial resolution using a classification tree approach. Int. J. Remote Sens. 2010, 21, 1331–1364. [Google Scholar] [CrossRef]
- Chan, J.C.-W.; Paelinckx, D. Evaluation of Random Forest and Adaboost tree-based ensemble classification and spectral band selection for ecotope mapping using airborne hyperspectral imagery. Remote Sens. Environ. 2008, 112, 2999–3011. [Google Scholar] [CrossRef]
- Waske, B.; Braun, M. Classifier ensembles for land cover mapping using multitemporal SAR imagery. ISPRS J. Photogramm. Remote Sens. 2009, 64, 450–457. [Google Scholar] [CrossRef]
- Guo, L.; Chehata, N.; Mallet, C.; Boukir, S. Relevance of airborne lidar and multispectral image data for urban scene classification using Random Forests. ISPRS J. Photogramm. Remote Sens. 2011, 66, 56–66. [Google Scholar] [CrossRef]
- Maxwell, A.E.; Strager, M.P.; Warner, T.A.; Ramezan, C.A.; Morgan, A.N.; Pauley, C.E. Large-Area, High Spatial Resolution Land Cover Mapping Using Random Forests, GEOBIA, and NAIP Orthophotography: Findings and Recommendations. Remote Sens. 2019, 11, 1409. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, A.E.; Strager, M.P.; Yuill, C.B.; Petty, J.T. Modeling Critical Forest Habitat in the Southern Coal Fields of West Virginia. Int. J. Ecol. 2012, 2012, 182683. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, A.E.; Warner, T.A.; Strager, M.P. Predicting Palustrine Wetland Probability Using Random Forest Machine Learning and Digital Elevation Data-Derived Terrain Variables. Photogramm. Eng. Remote Sens. 2016, 82, 437–447. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, A.; Welsh, W. Mapping Wetlands and Phragmites Using Publically Available Remotely Sensed Images. Photogramm. Eng. Remote Sens. 2015, 81, 69–78. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Pal, M.; Mather, P.M. An assessment of the effectiveness of decision tree methods for land cover classification. Remote Sens. Environ. 2003, 86, 554–565. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Lowe, B. Random Forest Algorithm for Land Cover Classification. 2016. Available online: https://scholarworks.uttyler.edu/compsci_fac/1/ (accessed on 16 December 2021).
- Mountrakis, G.; Im, J.; Ogole, C. Support vector machines in remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2011, 66, 247–259. [Google Scholar] [CrossRef]
- Pal, M. Random forest classifier for remote sensing classification. Int. J. Remote Sens. 2005, 26, 217–222. [Google Scholar] [CrossRef]
- Maxwell, A.E.; Warner, T.A.; Fang, F. Implementation of machine-learning classification in remote sensing: An applied review. Int. J. Remote Sens. 2018, 39, 2784–2817. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, B.; Rogan, J.; Rodriguez-Galiano, V.F.; Panday, P.; Neeti, N. An Evaluation of Bagging, Boosting, and Random Forests for Land-Cover Classification in Cape Cod, Massachusetts, USA. GIScience Remote Sens. 2012, 49, 623–643. [Google Scholar] [CrossRef]
- Hansen, M.; Dubayah, R.; DeFries, R. Classification trees: An alternative to traditional land cover classifiers. Int. J. Remote Sens. 1996, 17, 1075–1081. [Google Scholar] [CrossRef]
- Friedl, M.; Brodley, C. Decision tree classification of land cover from remotely sensed data. Remote Sens. Environ. 1997, 61, 399–409. [Google Scholar] [CrossRef]
- Rogan, J.; Miller, J.; Stow, D.; Franklin, J.; Levien, L.; Fischer, C. Land-Cover Change Monitoring with Classification Trees Using Landsat TM and Ancillary Data. Photogramm. Eng. Remote Sens. 2003, 69, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.M.; Miller, S.N.; Murphy, M. High-resolution landcover classification using Random Forest. Remote Sens. Lett. 2014, 5, 112–121. [Google Scholar] [CrossRef]
- Iv, J.F.E.; Michie, D.; Spiegelhalter, D.J.; Taylor, C.C. Machine Learning, Neural, and Statistical Classification. J. Am. Stat. Assoc. 1996, 91, 436. [Google Scholar] [CrossRef]
- Hughes, G. On the mean accuracy of statistical pattern recognizers. IEEE Trans. Inf. Theory 1968, 14, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Berhane, T.M.; Lane, C.R.; Wu, Q.; Autrey, B.C.; Anenkhonov, O.A.; Chepinoga, V.V.; Liu, H. Decision-Tree, Rule-Based, and Random Forest Classification of High-Resolution Multispectral Imagery for Wetland Mapping and Inventory. Remote Sens. 2018, 10, 580. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, J.M.; Knight, J.F.; Gallant, A.L. Influence of Multi-Source and Multi-Temporal Remotely Sensed and Ancillary Data on the Accuracy of Random Forest Classification of Wetlands in Northern Minnesota. Remote Sens. 2013, 5, 3212–3238. [Google Scholar] [CrossRef] [Green Version]
- Jerome, D.S. Landforms of the Upper Peninsula, Michigan; Natural Resources Conservation Service: Washington, DC, USA, 2006; p. 56. [Google Scholar]
- Wayne, W.J.; Zumberge, J.H. The Quaternary of the U.S. In Pleistocene Geology of Indiana and Michigan; Princeton University Press: Princeton, NJ, USA, 2015; pp. 63–84. [Google Scholar]
- Lessard, V.C.; McRoberts, R.E.; Holdaway, M.R. Diameter growth models using FIA data from the Northeastern, Southern, and North Central Research Stations. In Proceedings of the First Annual Forest Inventory and Analysis Symposium, San Antonio, TX, USA, 2–3 November 1999; Gen. Tech. Rep. NC-213; McRoberts, R.E., Reams, G.A., Van Deusen, P.C., Eds.; US Department of Agriculture, Forest Service, North Central Research Station: St. Paul, MN, USA, 2000; pp. 37–42. [Google Scholar]
- Archambault, L.; Barnes, B.V.; Witter, J.A. Ecological species groups of oak ecosystems of southeastern Michigan. For. Sci. 1989, 35, 1058–1074. [Google Scholar]
- Host, G.E.; Pregitzer, K.S. Ecological species groups for upland forest ecosystems of northwestern Lower Michigan. For. Ecol. Manag. 1991, 43, 87–102. [Google Scholar] [CrossRef]
- Zogg, G.P.; Barnes, B.V. Ecological classification and analysis of wetland ecosystems, northern Lower Michigan, USA. Can. J. For. Res. 1995, 25, 1865–1875. [Google Scholar] [CrossRef]
- Bhatt, P. Mapping Coastal Wetland and Phragmites on the Hiawatha National Forest Using Unmanned Aerial System (UAS) Imagery: Proof of Concepts; Michigan Technological University: Houghton, MI, USA, 2018. [Google Scholar]
- Jordan, J.K.; Padley, E.A.; Cleland, D.T. Landtype associations: Concepts and development in Lake States National Forests. In Proceedings Land Type Associations Conference: Development and Use in Natural Resources Management, Planning and Research; GTR-NE-294; USDA Forest Service: Newtown Square, PA, USA, 2001; pp. 11–23. [Google Scholar]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; R Core Team. Package ‘caret’. R J. 2020, 223, 7. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Adam, E.; Mutanga, O.; Rugege, D. Multispectral and hyperspectral remote sensing for identification and mapping of wetland vegetation: A review. Wetl. Ecol. Manag. 2010, 18, 281–296. [Google Scholar] [CrossRef]
- Whittaker, R.H. Classification of natural communities. Bot. Rev. 1962, 28, 1–239. [Google Scholar] [CrossRef]
- Lane, C.R.; Liu, H.; Autrey, B.C.; Anenkhonov, O.A.; Chepinoga, V.V.; Wu, Q. Improved Wetland Classification Using Eight-Band High Resolution Satellite Imagery and a Hybrid Approach. Remote Sens. 2014, 6, 12187–12216. [Google Scholar] [CrossRef] [Green Version]
- Akar, Ö.; Güngör, O. Classification of multispectral images using Random Forest algorithm. J. Geod. Geoinform. 2012, 1, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, A.E.; Warner, T.A.; Vanderbilt, B.C.; Ramezan, C.A. Land Cover Classification and Feature Extraction from National Agriculture Imagery Program (NAIP) Orthoimagery: A Review. Photogramm. Eng. Remote Sens. 2017, 83, 737–747. [Google Scholar] [CrossRef]
- Dunteman, G.H. Basic Concepts of Principal Components Analysis; SAGE Publications Ltd.: London, UK, 1989; pp. 15–22. [Google Scholar]
- Jensen, J.R. Introductory Digital Image Processing: A Remote Sensing Perspective; Prentice Hall Press: Glenview, IL, USA, 2015; p. 544. [Google Scholar]
- Munyati, C. Use of Principal Component Analysis (PCA) of Remote Sensing Images in Wetland Change Detection on the Kafue Flats, Zambia. Geocarto Int. 2004, 19, 11–22. [Google Scholar] [CrossRef]
- Dronova, I.; Gong, P.; Wang, L.; Zhong, L. Mapping dynamic cover types in a large seasonally flooded wetland using extended principal component analysis and object-based classification. Remote Sens. Environ. 2015, 158, 193–206. [Google Scholar] [CrossRef]
- Almeida, T.I.R.; Filho, D.S. Principal component analysis applied to feature-oriented band ratios of hyperspectral data: A tool for vegetation studies. Int. J. Remote Sens. 2004, 25, 5005–5023. [Google Scholar] [CrossRef]
- Lasaponara, R. On the use of principal component analysis (PCA) for evaluating interannual vegetation anomalies from SPOT/VEGETATION NDVI temporal series. Ecol. Model. 2006, 194, 429–434. [Google Scholar] [CrossRef]
- Kumar, C.; Chatterjee, S.; Oommen, T.; Guha, A. Automated lithological mapping by integrating spectral enhancement techniques and machine learning algorithms using AVIRIS-NG hyperspectral data in Gold-bearing granite-greenstone rocks in Hutti, India. Int. J. Appl. Earth Obs. Geoinform. 2020, 86, 102006. [Google Scholar] [CrossRef]
- Dwivedi, R.S.; Sankar, T.R. Principal component analysis of LANDSAT MSS data for delineation of terrain features. Int. J. Remote Sens. 1992, 13, 2309–2318. [Google Scholar] [CrossRef]
- Shah, C.A.; Arora, M.K.; Robila, S.A.; Varshney, P.K. ICA mixture model based unsupervised classification of hyperspectral imagery. In Proceedings of the Applied Imagery Pattern Recognition Workshop, 2002. Proceedings, Washington, DC, USA, 16–18 October 2002. [Google Scholar]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef] [Green Version]
- Shah, C.A.; Varshney, P.K.; Arora, M.K. ICA mixture model algorithm for unsupervised classification of remote sensing imagery. Int. J. Remote Sens. 2007, 28, 1711–1731. [Google Scholar] [CrossRef]
- Shah, C.A.; Anderson, I.; Gou, Z.; Hao, S.; Leason, A. Towards the development of next generation remote sensing technology–erdas imagine incorporates a higher order feature extraction technoque based on ica. In Proceedings of the ASPRS 2007 Annual Conference, Tampa, FL, USA, 7–11 May 2007. [Google Scholar]
- Li, F.; Xiao, B. Aquatic vegetation mapping based on remote sensing imagery: An application to Honghu Lake. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011. [Google Scholar]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Wolf, A.F. Using WorldView-2 Vis-NIR multispectral imagery to support land mapping and feature extraction using normalized difference index ratios. In Algorithms and Technologies for Multispectral, Hyperspectral, and Ultraspectral Imagery XVIII; International Society for Optics and Photonics: Baltimore, MD, USA, 2012. [Google Scholar]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Hall-Beyer, M. Practical guidelines for choosing GLCM textures to use in landscape classification tasks over a range of moderate spatial scales. Int. J. Remote Sens. 2017, 38, 1312–1338. [Google Scholar] [CrossRef]
- Maillard, P. Comparing Texture Analysis Methods through Classification. Photogramm. Eng. Remote Sens. 2003, 69, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Cleland, D.T.; Crow, T.R.; Avers, P.E.; Probst, J.R. Principles of Land Stratification for Delineating Ecosystems; Taking an Ecological Approach to Management; US Forest Service Watershed and Air Management: Salt Lake City, UT, USA, 1992; pp. 40–50. [Google Scholar]
- Cleland, D.; Shadis, D.; Dickman, D.I.; Jordan, J.K.; Watson, R. Use of Ecological Units in Mapping Natural Disturbance Regimes in the Lake States. In Proceedings of the Land Type Associations Conference: Development and Use in Natural Resources Management, Planning and Research, Madison, WI, USA, 24–26 April 2001. [Google Scholar]
- Hoque, N.; Bhattacharyya, D.K.; Kalita, J. MIFS-ND: A mutual information-based feature selection method. Expert Syst. Appl. 2014, 41, 6371–6385. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Bennasar, M.; Hicks, Y.; Setchi, R. Feature selection using Joint Mutual Information Maximisation. Expert Syst. Appl. 2015, 42, 8520–8532. [Google Scholar] [CrossRef] [Green Version]
- Kursa, M.B. Praznik: High performance information-based feature selection. SoftwareX 2021, 16, 100819. [Google Scholar] [CrossRef]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer: Berlin/Heidelberg, Germany, 2013; Volume 26. [Google Scholar]
- Löw, F.; Michel, U.; Dech, S.; Conrad, C. Impact of feature selection on the accuracy and spatial uncertainty of per-field crop classification using Support Vector Machines. ISPRS J. Photogramm. Remote Sens. 2013, 85, 102–119. [Google Scholar] [CrossRef]
- Laliberte, A.S.; Browning, D.; Rango, A. A comparison of three feature selection methods for object-based classification of sub-decimeter resolution UltraCam-L imagery. Int. J. Appl. Earth Obs. Geoinform. 2012, 15, 70–78. [Google Scholar] [CrossRef]
- Duro, D.C.; Franklin, S.; Dubé, M.G. Multi-scale object-based image analysis and feature selection of multi-sensor earth observation imagery using random forests. Int. J. Remote Sens. 2012, 33, 4502–4526. [Google Scholar] [CrossRef]
- Swain, P.H. Fundamentals of pattern recognition in remote sensing. In Remote Sensing: The Quantitative Approach; McGraw-Hill: New York, NY, USA, 1978; pp. 136–188. [Google Scholar]
- Huang, C.; Davis, L.S.; Townshend, J.R.G. An assessment of support vector machines for land cover classification. Int. J. Remote Sens. 2002, 23, 725–749. [Google Scholar] [CrossRef]
- Lu, D.; Weng, Q. A survey of image classification methods and techniques for improving classification performance. Int. J. Remote Sens. 2007, 28, 823–870. [Google Scholar] [CrossRef]
- Li, T.; Zhang, C.; Ogihara, M. A comparative study of feature selection and multiclass classification methods for tissue classification based on gene expression. Bioinformatics 2004, 20, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Brieman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regression Tree Analysis; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Breiman, L. Random Forests; UC Berkeley TR567; UC Berkeley: Berkeley, CA, USA, 1999. [Google Scholar]
- Swain, P.H.; Hauska, H. The decision tree classifier: Design and potential. IEEE Trans. Geosci. Electron. 1977, 15, 142–147. [Google Scholar] [CrossRef]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Segal, M.R. Machine Learning Benchmarks and Random Forest Regression; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. Random forests. In The Elements of Statistical Learning; Springer: Berlin/Heidelberg, Germany, 2009; pp. 587–604. [Google Scholar]
- Breiman, L.; Cutler, A. Random Forests-Classification Description; Department of Statistics: Berkeley, CA, USA, 2007; p. 2. [Google Scholar]
- Duro, D.; Franklin, S.; Dubé, M.G. A comparison of pixel-based and object-based image analysis with selected machine learning algorithms for the classification of agricultural landscapes using SPOT-5 HRG imagery. Remote Sens. Environ. 2012, 118, 259–272. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training. Astrophysics Source Code Library. 2015. Available online: https://ui.adsabs.harvard.edu/abs/2015ascl.soft05003K/abstract (accessed on 15 December 2021).
- Vapnik, V. The Nature of Statistical Learning Theory; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Yu, L.; Porwal, A.; Holden, E.-J.; Dentith, M. Towards automatic lithological classification from remote sensing data using support vector machines. Comput. Geosci. 2012, 45, 229–239. [Google Scholar] [CrossRef]
- Boser, B.E.; Guyon, I.M.; Vapnik, V.N. A training algorithm for optimal margin classifiers. In Proceedings of the Fifth Annual Workshop on Computational Learning Theory—COLT’92 , Pittsburgh, PA, USA, 27–29 July 1992; ACM Press: New York, NY, USA, 1992; pp. 144–152. [Google Scholar]
- Gunn, S.R. Support Vector Machines for Classification and Regression. ISIS Tech. Rep. 1998, 14, 5–16. [Google Scholar]
- Kuhn, M. The Caret Package. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://cran.r-project.org/package=caret (accessed on 21 July 2021).
- Kavzoglu, T.; Colkesen, I. A kernel functions analysis for support vector machines for land cover classification. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 352–359. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Chang, C.-C.; Lin, C.-J. A Practical Guide to Support Vector Classification; Department of Computer Science, National Taiwan University: Taipei, Taiwan, 2003. [Google Scholar]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the IJCAI, Montreal, QC, Canada, 20–25 August 1995. [Google Scholar]
- Fitzpatrick-Lins, K. Comparison of sampling procedures and data analysis for a land-use and land-cover map. Photogramm. Eng. Remote Sens. 1981, 47, 343–351. [Google Scholar]
- Rosenfield, G.H.; Fitzpatrick-Lins, K.; Ling, H. Sampling for thematic map accuracy testing. Photogramm. Eng. Remote Sens. 1982, 48, 131–137. [Google Scholar]
- Lillesand, T.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Congalton, R.G. A comparison of sampling schemes used in generating error matrices for assessing the accuracy of maps generated from remotely sensed data. Photogramm. Eng. Remote Sens. 1988, 54, 593–600. [Google Scholar]
- Macleod, R.D.; Congalton, R.G. A quantitative comparison of change-detection algorithms for monitoring eelgrass from remotely sensed data. Photogramm. Eng. Remote Sens. 1998, 64, 207–216. [Google Scholar]
- Adam, E.; Mutanga, O.; Odindi, J.; Abdel-Rahman, E.M. Land-use/cover classification in a heterogeneous coastal landscape using RapidEye imagery: Evaluating the performance of random forest and support vector machines classifiers. Int. J. Remote Sens. 2014, 35, 3440–3458. [Google Scholar] [CrossRef]










| Spectral Band Names | Wavelength (nm) | Spatial Resolution (cm) |
|---|---|---|
| Blue (B) | 435–495 nm | |
| Green (G) | 525–585 nm | 60 |
| Red (R) | 619–651 nm | |
| Near-Infrared (NIR) | 808–882 nm |
| Vegetation Community Type (Code) | Area (Hectares) | Training Data | Testing Data (Accuracy Assessment) |
|---|---|---|---|
| Open Land (OL) | 10.6 | 466 | 155 |
| Emergent Marsh (EM) | 64.7 | 3205 | 1068 |
| Rich Conifer Swamp (RCS) | 202.5 | 4057 | 1352 |
| Poor Conifer Swamp (PCS) | 142.9 | 2272 | 757 |
| Northern Shrub Thicket (NST) | 54.9 | 2620 | 873 |
| Mesic Northern Forest (MNF) | 108.3 | 3814 | 1271 |
| Inland Lake (IL) | 8.3 | 386 | 128 |
| Open Water (OW) | 5.3 | 292 | 97 |
| Impervious Surfaces (IS) | 5.5 | 301 | 100 |
| Total | 603 | 17,413 | 5801 |
| Input Data | Variable Combinations | OA (%) (RF) | k (RF) | OA (%) (SVM) | k (SVM) |
|---|---|---|---|---|---|
| 1 | NAIP bands | 56.02 | 0.46 | 59.01 | 0.49 |
| 2 | NAIP bands + DEM | 73.09 | 0.67 | 71.21 | 0.65 |
| 3 | NAIP + DEM + Asp+ Slp + Tex + NDVI + WINAIP + Tex2 + Tex3 | 79.96 | 0.75 | 75.35 | 0.70 |
| 4 | NAIP + DEM + Tex1 + NDVI + WINAIP (JMIM based optimal input variables) | 79.45 | 0.75 | 75.85 | 0.70 |
| Input Number | Variable Combinations | k (RF) | Z-Score (RF) | k (SVM) | Z-Score (SVM) | Z-Score (Pairwise) | 95% CI (RF) | 95% CI (SVM) |
|---|---|---|---|---|---|---|---|---|
| 1 | NAIP bands | 0.46 | 2.37 | 0.49 | 2.72 | −0.34 | ±1.28 | ±1.27 |
| 2 | NAIP bands + DEM | 0.67 | 5.02 | 0.65 | 5.09 | 0.196 | ±1.15 | ±1.17 |
| 3 | NAIP + DEM + Asp + Slp + Tex + NDVI + WINAIP + Tex2+ Tex3 | 0.75 | 6.29 | 0.70 | 6.13 | 0.467 | ±1.02 | ±1.11 |
| 4 | NAIP + DEM + Tex1 + NDVI + WINAIP | 0.75 | 6.29 | 0.70 | 6.13 | 0.467 | ±1.04 | ±1.10 |
| RF | OL | EM | RCS | PCS | NST | MNF | IL | OW | IS | Total | UA (%) | PA (%) |
| OL | 146 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 148 | 98.64 | 94.19 |
| EM | 0 | 940 | 20 | 8 | 61 | 10 | 0 | 0 | 0 | 1039 | 90.47 | 88.01 |
| RCS | 0 | 42 | 1053 | 275 | 128 | 53 | 0 | 0 | 1 | 1552 | 67.85 | 77.88 |
| PCS | 0 | 10 | 176 | 418 | 23 | 17 | 0 | 1 | 1 | 646 | 64.70 | 55.22 |
| NST | 0 | 59 | 81 | 37 | 592 | 47 | 0 | 0 | 0 | 816 | 72.55 | 67.81 |
| MNF | 9 | 14 | 22 | 19 | 69 | 1142 | 0 | 0 | 3 | 1278 | 89.36 | 89.85 |
| IL | 0 | 1 | 0 | 0 | 0 | 0 | 128 | 0 | 0 | 129 | 99.22 | 100.00 |
| OW | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 96 | 0 | 98 | 97.96 | 98.97 |
| IS | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 94 | 95 | 98.95 | 94.00 |
| Total | 155 | 1068 | 1352 | 757 | 873 | 1271 | 128 | 97 | 100 | 5801 | OA = 79.45%, k = 0.75 | |
| SVM | OL | EM | RCS | PCS | NST | MNF | IL | OW | IS | Total | UA (%) | PA (%) |
| OL | 145 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 146 | 99.31 | 93.55 |
| EM | 0 | 898 | 21 | 6 | 66 | 7 | 0 | 0 | 1 | 999 | 89.88 | 84.08 |
| RCS | 0 | 71 | 1101 | 371 | 196 | 93 | 0 | 0 | 0 | 1832 | 60.09 | 81.43 |
| PCS | 0 | 3 | 139 | 314 | 11 | 11 | 0 | 1 | 1 | 480 | 65.41 | 41.48 |
| NST | 0 | 81 | 72 | 35 | 526 | 43 | 0 | 0 | 0 | 757 | 69.48 | 60.25 |
| MNF | 10 | 12 | 19 | 31 | 74 | 1116 | 0 | 0 | 8 | 1270 | 87.87 | 87.80 |
| IL | 0 | 0 | 0 | 0 | 0 | 0 | 128 | 14 | 0 | 142 | 90.14 | 100.00 |
| OW | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 82 | 0 | 85 | 96.47 | 84.53 |
| IS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 90 | 100.00 | 90.00 |
| Total | 155 | 1068 | 1352 | 757 | 873 | 1271 | 128 | 97 | 100 | 5801 | OA = 75.85%, k = 0.70 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, P.; Maclean, A.; Dickinson, Y.; Kumar, C. Fine-Scale Mapping of Natural Ecological Communities Using Machine Learning Approaches. Remote Sens. 2022, 14, 563. https://doi.org/10.3390/rs14030563
Bhatt P, Maclean A, Dickinson Y, Kumar C. Fine-Scale Mapping of Natural Ecological Communities Using Machine Learning Approaches. Remote Sensing. 2022; 14(3):563. https://doi.org/10.3390/rs14030563
Chicago/Turabian StyleBhatt, Parth, Ann Maclean, Yvette Dickinson, and Chandan Kumar. 2022. "Fine-Scale Mapping of Natural Ecological Communities Using Machine Learning Approaches" Remote Sensing 14, no. 3: 563. https://doi.org/10.3390/rs14030563