Predicting Water Stress in Wild Blueberry Fields Using Airborne Visible and Near Infrared Imaging Spectroscopy
Abstract
:1. Introduction
- Collecting airborne data on an irrigated and non-irrigated field over three plant development stages.
- Acquire water potentials of canopy leaf samples in each field.
- Generate classified maps of irrigated vs. non-irrigated areas and estimated water potentials to determine locations of low or high plant canopy water stress.
2. Materials and Methods
2.1. Study Site
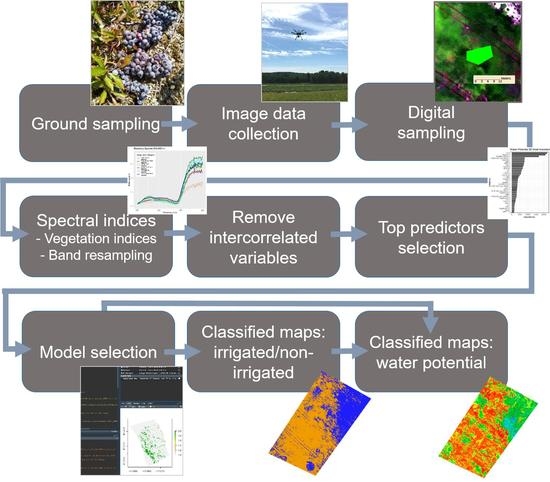
2.2. Workflow Overview
2.3. Ground Sampling
2.4. Image Data Collection and Sampling
2.5. Model Development
3. Results
3.1. Model
3.2. Validation
3.3. Variable Importance
3.4. Spectral Signatures
3.5. Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, M.B.; Mcgonigle, A.J.S.; Willmott, J.R. Hyperspectral Imaging in Environmental Monitoring: A Review of Recent Developments and Technological Advances in Compact Field Deployable Systems. Sensors 2019, 19, 3071. [Google Scholar] [CrossRef] [Green Version]
- Green, R.O.; Eastwood, M.L.; Sarture, C.M.; Chrien, T.G.; Aronsson, M.; Chippendale, B.J.; Faust, J.A.; Pavri, B.E.; Chovit, C.J.; Solis, M.; et al. Imaging Spectroscopy and the Airborne Visible/Infrared Imaging Spectrometer (AVIRIS). Remote Sens. Environ. 1998, 65, 227–248. [Google Scholar] [CrossRef]
- Marrs, J.; Ni-Meister, W. Machine Learning Techniques for Tree Species Classification Using Co-Registered LiDAR and Hyperspectral Data. Remote Sens. 2019, 11, 819. [Google Scholar] [CrossRef] [Green Version]
- Nguyen-Do-Trong, N.; Dusabumuremyi, J.C.; Saeys, W. Cross-polarized VNIR hyperspectral reflectance imaging for non-destructive quality evaluation of dried banana slices, drying process monitoring and control. J. Food Eng. 2018, 238, 85–94. [Google Scholar] [CrossRef]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2017, 125, 5–20. [Google Scholar] [CrossRef]
- Houborg, R.; Fisher, J.B.; Skidmore, A.K. Advances in remote sensing of vegetation function and traits. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wu, H.; Wang, L.; Huang, B.; Ranjan, R.; Zomaya, A.; Jie, W. Remote sensing big data computing: Challenges and opportunities. Futur. Gener. Comput. Syst. 2015, 51, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trends Ecol. Evol. 2003, 18, 299–305. [Google Scholar] [CrossRef]
- Koch, B. Status and future of laser scanning, synthetic aperture radar and hyperspectral remote sensing data for forest biomass assessment. ISPRS J. Photogramm. Remote Sens. 2010, 65, 581–590. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ehsani, R.; Abd-Elrahman, A.; Ampatzidis, Y. A remote sensing technique for detecting laurel wilt disease in avocado in presence of other biotic and abiotic stresses. Comput. Electron. Agric. 2019, 156, 549–557. [Google Scholar] [CrossRef]
- Rahman, M.R.; Islam, A.H.M.H.; Rahman, M.A. NDVI derived sugarcane area identification and crop condition assessment. Plan Plus 2004, 1, 1–12. [Google Scholar]
- Asner, G.P.; Nepstad, D.C.; Cardinot, G.A.; Ray, D. From The Cover: Drought stress and carbon uptake in an Amazon forest measured with spaceborne imaging spectroscopy. Proc. Natl. Acad. Sci. USA 2004, 101, 6039–6044. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.-A. Understanding water deficit stress-induced changes in the basic metabolism of higher plants–biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Recent advances in crop water stress detection. Comput. Electron. Agric. 2017, 141, 267–275. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Robbins, N.E.; Dinneny, J.R. The divining root: Moisture-driven responses of roots at the micro- and macro-scale. J. Exp. Bot. 2015, 66, 2145–2154. [Google Scholar] [CrossRef] [Green Version]
- Améglio, T.; Archer, P.; Cohen, M.; Valancogne, C.; Daudet, F.-A.; Dayau, S.; Cruiziat, P. Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant Soil 1998, 207, 155–167. [Google Scholar] [CrossRef]
- Jones, H.G. Physiological Aspects of the Control of Water Status in Horticultural Crops. HortScience 1990, 25, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Borengasser, M.; Hungate, W.S.; Watkins, R. Hyperspectral Remote Sensing: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Rose, A.; Drummond, F.A.; Yarborough, D.E.; Asare, E. MR445: Maine Wild Blueberry Growers: A 2010 Economic and Sociological Analysis of a Traditional Downeast Crop in Transition; DigitalCommons@UMaine: Orono, ME, USA, 2013. [Google Scholar]
- Glass, V.M.; Percival, D.C. Proctor tolerance of lowbush blueberries (Vaccinium angustifolium Ait.) to drought stress. II. Soil water and yield component analysis. Can. J. Plant Sci. 2005, 85, 911–927. [Google Scholar] [CrossRef]
- Obsie, E.Y.; Qu, H.; Drummond, F. Wild blueberry yield prediction using a combination of computer simulation and machine learning algorithms. Comput. Electron. Agric. 2020, 178, 105778. [Google Scholar] [CrossRef]
- Tasnim, R.; Drummond, F.; Zhang, Y.-J. Climate Change Patterns of Wild Blueberry Fields in Downeast, Maine over the Past 40 Years. Water 2021, 13, 594. [Google Scholar] [CrossRef]
- Whittle, P. Hard Times as Maine Wild Blueberry Industry in Decline. 2018. Available online: https://www.timesrecord.com/articles/maine-1/hard-times-as-maine-wild-blueberry-industry-in-decline/ (accessed on 24 November 2018).
- Tasnim, R.; Calderwood, L.; Annis, S.; Drummond, F.A.; Zhang, Y.J. The Future of Wild Blueberries: Testing Warming Impacts Using Open-Top Chambers; Spire: Orono, ME, USA, 2020. [Google Scholar]
- Johnston, D.W. Unoccupied aircraft systems in marine science and conservation. Annu. Rev. Mar. Sci. 2019, 11, 439–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Sun, J.; Mao, H.; Wu, X.; Zhang, X.; Yang, N. Visualization research of moisture content in leaf lettuce leaves based on WT-PLSR and hyperspectral imaging technology. J. Food Process Eng. 2018, 41, e12647. [Google Scholar] [CrossRef]
- Rapaport, T.; Hochberg, U.; Shoshany, M.; Karnieli, A.; Rachmilevitch, S. Combining leaf physiology, hyperspectral imaging and partial least squares-regression (PLS-R) for grapevine water status assessment. ISPRS J. Photogramm. Remote Sens. 2015, 109, 88–97. [Google Scholar] [CrossRef]
- Nelson, P.R. Github Repository; LECOSPEC: San Francisco, CA, USA, 2020; Available online: https://github.com/nelsopet/lecospec (accessed on 28 February 2021).
- Nelson, P.; Thompson, N. Visible and Infrared Imaging Spectroscopy for High Resolution Mapping and Health Assessment of Maine’s Forest and Agricultural Resources; Maine Economic Improvement Funds (MEIF) Small Campus Initiative (SCI) Research Grant: Orono, ME, USA, 2018. [Google Scholar]
- Anthony, A.; Imke, D.; Scott, A.; Mike, S.; Russell, V.; Xungang, Y.; Rocky, B. NOAA’s U.S. Climate Normals (1981–2010); NOAA National Centers for Environmental Information: Asheville, NC, USA. [CrossRef]
- Farooque, A.A.; Zaman, Q.U.; Schumann, A.W.; Madani, A.; Percival, D.C. Response of wild blueberry yield to spatial variability of soil properties. Soil Sci. 2012, 177, 56–68. [Google Scholar] [CrossRef]
- Annis, S.L.; Stubbs, C.S. Stem and Leaf Diseases and Their Effects on Yield in Maine Lowbush Blueberry Fields. Small Fruits Rev. 2004, 3, 159–167. [Google Scholar] [CrossRef]
- Alghory, A.; Yazar, A. Evaluation of crop water stress index and leaf water potential for deficit irrigation management of sprinkler-irrigated wheat. Irrig. Sci. 2019, 37, 61–77. [Google Scholar] [CrossRef]
- Forkuor, G.; Hounkpatin, O.K.L.; Welp, G.; Thiel, M. High Resolution Mapping of Soil Properties Using Remote Sensing Variables in South-Western Burkina Faso: A Comparison of Machine Learning and Multiple Linear Regression Models. PLoS ONE 2017, 12, e0170478. [Google Scholar]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Schilder, A. Growth Stages. Michigan State University Extension. April 2015. Available online: https://www.canr.msu.edu/blueberries/growing_blueberries/growth-stages (accessed on 20 November 2018).
- Hepler, P.R.; Yarborough, D.E. Natural Variability in Yield of Lowbush Blueberries. HortScience 1991, 26, 245–246. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.J.; Ustin, S.L.; Whiting, M.L. Temporal and Spatial Relationships between Within-Field Yield Variability in Cotton and High-Spatial Hyperspectral Remote Sensing Imagery. Agron. J. 2005, 97, 641–653. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar]
- Calderón, R.; Navas-Cortés, J.A.; Zarco-Tejada, P.J. Early detection and quantification of Verticillium wilt in olive using hyperspectral and thermal imagery over large areas. Remote Sens. 2015, 7, 5584–5610. [Google Scholar] [CrossRef] [Green Version]
- Poona, N.; Van Niekerk, A.; Ismail, R. Investigating the utility of oblique tree-based ensembles for the classification of hyperspectral data. Sensors 2016, 16, 1918. [Google Scholar] [CrossRef] [Green Version]
- Adjorlolo, C.; Mutanga, O.; Cho, M.A.; Ismail, R. Spectral resampling based on user-defined inter-band correlation filter: C3 and C4 grass species classification. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 535–544. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Meyer, H.; Obermeier, W.A.; Silva, B.; Regeling, B.; Bendix, J. Hyperspectral Data Analysis in R: The hsdar Package. J. Stat. Softw. 2019, 89, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Boonprong, S.; Cao, C.; Chen, W.; Bao, S. Random forest variable importance spectral indices scheme for burnt forest recovery monitoring—Multilevel RF-VIMP. Remote Sens. 2018, 10, 807. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.N.; Ziegler, A. Ranger: A fast implementation of random forests for high dimensional data in C++ and R. J. Stat. Softw. 2017, 77, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Rinnan, Å.; Bruun, S.; Lindedam, J.; Decker, S.R.; Turner, G.B.; Felby, C.; Engelsen, S.B. Predicting the ethanol potential of wheat straw using near-infrared spectroscopy and chemometrics: The challenge of inherently intercorrelated response functions. Anal. Chim. Acta 2017, 962, 15–23. [Google Scholar] [CrossRef]
- Ludovisi, R.; Tauro, F.; Salvati, R.; Khoury, S.; Mugnozza Scarascia, G.; Harfouche, A. UAV-based thermal imaging for high-throughput field phenotyping of black poplar response to drought. Front. Plant Sci. 2017, 8, 1681. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, J.; Xu, X.; Liang, D.; Yang, G.; Feng, H.; Yang, H.; Wang, Y.; Chen, G.; Wei, P. Hyperspectral-based estimation of leaf nitrogen content in corn using optimal selection of multiple spectral variables. Sensors 2019, 19, 2898. [Google Scholar] [CrossRef] [Green Version]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Gitelson, A.; Buschmann, C.; Lichtenthaler, H.K. The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of the Chlorophyll Content in Plants. Remot Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Vidhya, R.; Vijayasekaran, D.; Farook, M.A.; Jai, S.; Rohini, M.; Sinduja, A. Improved Classification of Mangroves Health Status Using Hyperspectral Remote Sensing Data. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, XL-8, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Ge, X.; Wang, J.; Ding, J.; Cao, X.; Zhang, Z.; Liu, J.; Li, X. Combining UAV-based hyperspectral imagery and machine learning algorithms for soil moisture content monitoring. PeerJ 2019, 7, e6926. [Google Scholar] [CrossRef]
- Liang, L.; Di, L.; Zhang, L.; Deng, M.; Qin, Z.; Zhao, S.; Lin, H. Estimation of crop LAI using hyperspectral vegetation indices and a hybrid inversion method. Remote Sens. Environ. 2015, 165, 123–134. [Google Scholar] [CrossRef]
- Rumpf, T.; Mahlein, A.-K.; Steiner, U.; Oerke, E.-C.; Dehne, H.-W.; Plümer, L. Early detection and classification of plant diseases with Support Vector Machines based on hyperspectral reflectance. Comput. Electron. Agric. 2010, 74, 91–99. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2006, 58, 119–130. [Google Scholar]
- Chaerle, L.; Lenk, S.; Leinonen, I.; Jones, H.G.; Van Der Straeten, D.; Buschmann, C. Multi-sensor plant imaging: Towards the development of a stress-catalogue. Biotechnol. J. 2009, 4, 1152–1167. [Google Scholar] [CrossRef] [PubMed]
- Panta, G.; Rieger, M.; Rowland, L. Effect of cold and drought stress on blueberry dehydrin accumulation. J. Hortic. Sci. Biotechnol. 2001, 76, 549–556. [Google Scholar] [CrossRef]
- Zovko, M.; Žibrat, U.; Knapič, M.; Kovačić, M.B.; Romić, D. Hyperspectral remote sensing of grapevine drought stress. Precis. Agric. 2019, 20, 335–347. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Meron, M.; Saranga, Y.; Tsipris, J. Estimation of leaf water potential by thermal imagery and spatial analysis. J. Exp. Bot. 2005, 56, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Thorp, K.; Wang, G.; Bronson, K.; Badaruddin, M.; Mon, J. Hyperspectral data mining to identify relevant canopy spectral features for estimating durum wheat growth, nitrogen status, and grain yield. Comput. Electron. Agric. 2017, 136, 1–12. [Google Scholar] [CrossRef] [Green Version]
- González-Fernández, A.B.; Sanz-Ablanedo, E.; Gabella, V.M.; García-Fernández, M.; Rodríguez-Pérez, J.R. Field Spectroscopy: A Non-Destructive Technique for Estimating Water Status in Vineyards. Agronomy 2019, 9, 427. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Peng, Y.; Du, W.; Le, Y.; Li, L. Remote Estimation of Leaf and Canopy Water Content in Winter Wheat with Different Vertical Distribution of Water-Related Properties. Remote Sens. 2015, 7, 4626–4650. [Google Scholar] [CrossRef] [Green Version]
- Osco, L.P.; Ramos, A.P.M.; Pinheiro, M.M.F.; Moriya, É.A.S.; Imai, N.N.; Estrabis, N.; Ianczyk, F.; De Araújo, F.F.; Liesenberg, V.; Jorge, L.A.D.C.; et al. A Machine Learning Framework to Predict Nutrient Content in Valencia-Orange Leaf Hyperspectral Measurements. Remote Sens. 2020, 12, 906. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhou, G. Estimation of vegetation water content using hyperspectral vegetation indices: A comparison of crop water indicators in response to water stress treatments for summer maize. BMC Ecol. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pôças, I.; Gonçalves, J.; Costa, P.M.; Gonçalves, I.; Pereira, L.S.; Cunha, M. Hyperspectral-based predictive modelling of grapevine water status in the Portuguese Douro wine region. Int. J. Appl. Earth Obs. Geoinform. 2017, 58, 177–190. [Google Scholar] [CrossRef]
- Scialabba, N. Factors influencing organic agriculture policies with a focus on developing countries. In Proceedings of the IFOAM 2000 Scientific Conference, Basel, Switzerland, 28–31 August 2000; Volume 28, p. 31. [Google Scholar]
- Ramoelo, A.; Dzikiti, S.; Van Deventer, H.; Maherry, A.; Cho, M.A.; Gush, M. Potential to monitor plant stress using remote sensing tools. J. Arid. Environ. 2015, 113, 134–144. [Google Scholar] [CrossRef]
- McBratney, A.; Whelan, B.; Ancev, T.; Bouma, J. Future Directions of Precision Agriculture. Precis. Agric. 2005, 6, 7–23. [Google Scholar] [CrossRef]







| Irrigated/Non-Irrigated Local Classification | Water Potential Local Regression | Water Potential Global Regression | |||||
|---|---|---|---|---|---|---|---|
| Peak Bloom | Green Fruit | Color Break | Peak Bloom | Green Fruit | Color Break | ||
| Sample Size (pixels) | 47,758 | 32,018 | 103,135 | 139 | 115 | 99 | 353 |
| Independent variables | 25 | 25 | 25 | 15 | 25 | 25 | 20 |
| Out-of-bag (OOB) prediction error/MSE | 1.346% | 0.003% | 1.402% | 320 | 679 | 754 | 709 |
| R2 (OOB) | NA | 0.487 | 0.458 | 0.437 | 0.554 | ||
| Validation Metric | Local Regression Prediction | Global Regression Prediction | ||
|---|---|---|---|---|
| Peak Bloom | Green Fruit | Color Break | ||
| OOB prediction error (MSE) | 237 | 533 | 556 | 616 |
| R2 (OOB) | 0.554 | 0.563 | 0.564 | 0.617 |
| Calculated RSME | 29.8 | 36.9 | 45.2 | 46.4 |
| Abbreviation | Name | Formula | |
|---|---|---|---|
| 1 | Datt | ‘Chlorophyll & height’ | (R749 − R720) − (R701 − R672) |
| 2 | Gitelson | ‘Chlorophyll’ | 1/R700 |
| 3 | TCARI2OSAVI2 | Transformed Chlorophyll Absorption Ratio 2/Optimized Soil Adjusted Vegetation Index 2 | (3 * ((R750 − R705) − 0.2 * (R750 −R550) * (R750/R705)))/ ((1 + 0.16) * (R750−R705)/(R750 + R705 + 0.16)) |
| 4 | X692.593_wvl_005nm | ‘Bandpass 692.593 resampled at 5 nm’ | |
| 5 | Maccioni | ‘Chlorophyll’ | (R780 − R710)/(R780 − R680) |
| 6 | Datt2 | ‘Chlorophyll & height’ | R850/R710 |
| 7 | MTCI | MERIS Terrestrial Chlorophyll Index | (R754 − R709)/(R709 − R681) |
| 8 | DD | Double Difference Index | (R749 − R720) − (R701 − R672) |
| 9 | DWSI4 | Disease Water Stress Index 4 | R550/R680 |
| 10 | GDVI4 | Green Difference Vegetation Index 4 | (R4800 − R4680)/(R4800 + R4680) |
| 11 | GI | Greenness Index | R554/R677 |
| 12 | CARI | Chlorophyll Absorption Ration Index | R700 * abs(a * 670 + R670 + b)/R670 * (α2 + 1)0.5 α = (R700 − R550)/150 b = R550 − (550 *α) |
| 13 | TCARI2 | Transformed Chlorophyll Absorption Ratio 2 | (3 * ((R750 − R705) − 0.2 * (R750 − R550) * (R750/R705))) |
| 14 | Boochs2 | Single Band 703 Boochs | D703 |
| 15 | mSR | modified Simple Ratio | (R800 − R445)/(R680 − R445) |
| 16 | Ctr5 | Carter 5 | R695/R670 |
| 17 | EVI | Enhanced Vegetation Index | 2.5 * ((R800 − R670)/(R800 − (6 * R670) − (7.5 * R475) + 1)) |
| 18 | SumDr1 | ‘LAI & % green cover’ | |
| 19 | TCARIOSAVI | Transformed Chlorophyll Absorption Ratio/Optimized Soil Adjusted Vegetation Index | (3 * ((R700 − R670) − 0.2 * (R700 − R550) * (R700/R670)))/ ((1 + 0.16) * (R800 − R670)/(R800 + R670 + 0.16)) |
| 20 | X397.593_wvl_100_nm | ‘Bandpass 397.593 resampled at 100 nm’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.; Nelson, P.R.; Hayes, D.J.; Zhang, Y.-J.; Hall, B. Predicting Water Stress in Wild Blueberry Fields Using Airborne Visible and Near Infrared Imaging Spectroscopy. Remote Sens. 2021, 13, 1425. https://doi.org/10.3390/rs13081425
Chan C, Nelson PR, Hayes DJ, Zhang Y-J, Hall B. Predicting Water Stress in Wild Blueberry Fields Using Airborne Visible and Near Infrared Imaging Spectroscopy. Remote Sensing. 2021; 13(8):1425. https://doi.org/10.3390/rs13081425
Chicago/Turabian StyleChan, Catherine, Peter R. Nelson, Daniel J. Hayes, Yong-Jiang Zhang, and Bruce Hall. 2021. "Predicting Water Stress in Wild Blueberry Fields Using Airborne Visible and Near Infrared Imaging Spectroscopy" Remote Sensing 13, no. 8: 1425. https://doi.org/10.3390/rs13081425