Insect Target Classes Discerned from Entomological Radar Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radar Observations
2.2. Weather
3. Results
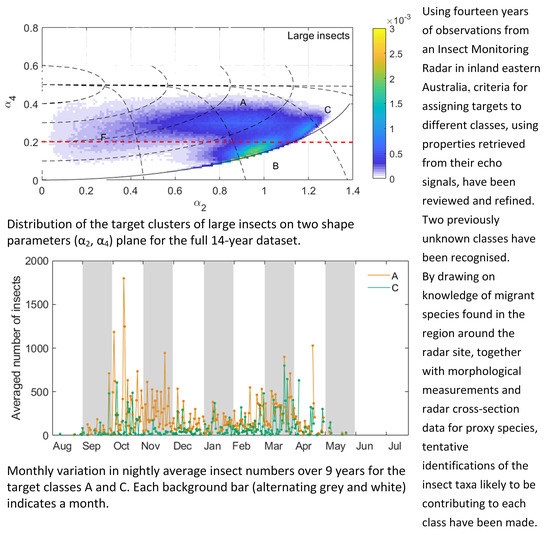
3.1. Clustering Analysis
3.2. Flight Temperature
3.3. Year-to-Year and Seasonal Variations
4. Identity of Species Forming the Target Classes
4.1. Available Resources that Could Help with Identification of Classes
4.1.1. RCS and CLPP Data of Proxy Species
4.1.2. Aerial-Netting and Light-Trap Catches
4.1.3. Australian Morphological Data
4.2. Identity of Species Forming the Target Classes
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| N | Mean (mg) | Std (mg) | Range (mg) | σxx/σyy | |
|---|---|---|---|---|---|
| Noctuid moths | |||||
| Autographa gamma [44] | 11 | 146 | 35 | 111–181 | |
| Noctua pronuba [15] | 33 | 344 | 66 | 300–500 | |
| Sphingid moths | |||||
| Agrius convolvuli [15] | 1 | 1212 | 600–1500 1 | ||
| Sphinx igustri [15] | 7 | 1256 | 146 | ||
| Laothoe populi [15] | 18 | 658 | 155 | ||
| Deilephila elpenor [15] | 13 | 595 | 68 | ||
| Hyloicus pinastri [15] | 5 | 571 | 164 | ||
| Nymphalid butterflies | |||||
| Vanessa atalanta [15] | 7 | 272 | 60 | 100–400 2 | |
| Vanessa cardui [15] | 17 | 192 | 56 | ||
| Ladybird beetles | |||||
| Harmonia axyridis and Coccinella septempunctata [13] | 20/10 | - | - | 25–42 2 | 1.5–4.1 |
| Hoverflies | |||||
| Episyrphus balteatus and Eupeodes corollae [14] | 18 | 22.3 | 6.6 | 15–28 2 | 5–10 |
| Target | a0 (cm2) | α4 | fw (Hz) | Mass (mg) |
|---|---|---|---|---|
| (~0.001–~10 cm2) | (0–~0.5) | (0–~130) | ||
| large moths | 0.5 ≤ a0 < 5 | α4 < 0.2 | 21 < fw < 50 | 100–2572 |
| C. terminifera | 0.5 ≤ a0 < 5 | α4 ≥ 0.2 | 21 < fw < 35 | 100–950 |
| medium moth | 0.05 ≤ a0 < 0.5 | α4 < 0.2 | 21 < fw < 50 | 40–94 |
| small insects | 0.005 < a0 < 0.05 | - | - | 10–28 |
| Class | a0 (dBsc) | p | q | fw (Hz) |
|---|---|---|---|---|
| LL810 | −3.3, 6.7 | 0.58, 0.83 | 0.73, 1.28 | 22.6, 30.3 |
| LL1013 | −3.3, 3.3 | 0.92, 1 | 1.1, 1.47 | 22.6, 39 |
| LL65 | 0, 6.7 | 0.5, 0.75 | 0.18, 0.73 | 22.6, 30.3 |
| ML/MM | −10, 0 | 0.92, 1 | 0.92, 1.1 | 22.6, 49 |
| MvHM | −10, −3.3 | 0.92, 1 | 0.92, 1.1 | 60, 86 |
| SMM | −20, −10 | 0.92, 1 | 0.92, 1.28 | 22.6, 49 |
Equations
References
- Drake, V.A.; Reynolds, D.R. Radar Entomology: Observing Insect Flight and Migration; CABI: New York, NY, USA, 2012. [Google Scholar]
- Chapman, J.W.; Drake, V.A.; Reynolds, D.R. Recent insights from radar studies of insect flight. Annu. Rev. Entomol. 2011, 56, 337–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, V.A.; Wang, H.K.; Harman, I.T. Insect monitoring radar: Remote and network operation. Comput. Electron. Agric. 2002, 35, 77–94. [Google Scholar] [CrossRef]
- Drake, V.A.; Hatty, S.; Symons, C.; Wang, H. Insect Monitoring Radar: Maximizing Performance and Utility. Remote Sens. 2020, 12, 596. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.H.; Drake, V.A.; Sidhu, L.; Taylor, J.R. Locust displacing winds in eastern Australia reassessed with observations from an insect monitoring radar. Int. J. Biometeorol. 2017, 61, 2073–2084. [Google Scholar] [CrossRef]
- Otuka, A.; Matsumura, M.; Tokuda, M. Development of method to forecast soybean leaf damage by common cutworm using entomological radar and searchlight trap. J. Eng. 2019, 2019, 7531–7533. [Google Scholar] [CrossRef]
- Wang, H.K. Evaluation of Insect Monitoring Radar Technology for Monitoring Locust Migrations in Inland Eastern Australia. Ph.D. Thesis, The University of New South Wales, Canberra, Australia, 2008. [Google Scholar]
- Drake, V.A.; Wang, H.K. Recognition and characterization of migratory movements of Australian plague locusts, Chortoicetes terminifera, with an insect monitoring radar. J. Appl. Remote Sens. 2013, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.W.; Smith, A.D.; Woiwod, I.P.; Reynolds, D.R.; Riley, J.R. Development of vertical-looking radar technology for monitoring insect migration. Comput. Electron. Agric. 2002, 35, 95–110. [Google Scholar] [CrossRef]
- Smith, A.D.; Riley, J.R.; Gregory, R.D. A method for routine monitoring of the aerial migration of insects by using a vertical-looking radar. Philos. Trans. R. Soc. B 1993, 340, 393–404. [Google Scholar] [CrossRef]
- Wang, H.K.; Drake, V.A. Insect monitoring radar: Retrieval of wingbeat information from conical-scan observation data. Comput. Electron. Agric. 2004, 43, 209–222. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Brooks, S.J.; Smith, A.D.; Woiwod, I.P. Seasonal variation in the migration strategies of the green lacewing Chrysoperla carnea species complex. Ecol. Entomol. 2006, 31, 378–388. [Google Scholar] [CrossRef]
- Jeffries, D.L.; Chapman, J.W.; Roy, H.E.; Humphries, S.; Harrington, R.; Brown, P.M.; Handley, L.J. Characteristics and drivers of high-altitude ladybird flight: Insights from vertical-looking entomological radar. PLoS ONE 2013, 8, e82278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wotton, K.R.; Gao, B.; Menz, M.H.M.; Morris, R.K.A.; Ball, S.G.; Lim, K.S.; Reynolds, D.R.; Hu, G.; Chapman, J.W. Mass seasonal migrations of hoverflies provide extensive pollination and crop protection services. Curr. Biol. 2019, 29, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; Nesbit, R.L.; Burgin, L.E.; Reynolds, D.R.; Smith, A.D.; Middleton, D.R.; Hill, J.K. Flight orientation behaviors promote optimal migration trajectories in high-flying insects. Science 2010, 327, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.R.; Reynolds, D.R. Radar-based studies of the migratory flight of grasshoppers in the middle Niger area of Mali. Proc. R. Soc. Lond. B Biol. Sci. 1979, 204, 67–82. [Google Scholar]
- Drake, V.A.; Farrow, R.A. A radar and aerial-trapping study of an early spring migration of moths (Lepidoptera) in inland New South Wales. Aust. J. Ecol. 1985, 10, 223. [Google Scholar] [CrossRef]
- Gregg, P.C.; Fitt, G.P.; Coombs, M.; Henderson, G.S. Migrating moths collected in tower-mounted light traps in northern New South Wales, Australia: Influence of local and synoptic weather. Bull. Entomol. Res. 1994, 84, 17–30. [Google Scholar] [CrossRef]
- Locust Bulletin, Australian Plague Locust Commission, Australia. Available online: https://www.agriculture.gov.au/pests-diseases-weeds/locusts/bulletins (accessed on 15 October 2019).
- Gregg, P. Pollen as a marker for migration of Helicoverpa armigera and H. punctigera (Lepidoptera: Noctuidae) from western Queensland. Aust. J. Ecol. 1993, 18, 209–219. [Google Scholar] [CrossRef]
- Warrant, E.; Frost, B.; Green, K.; Mouritsen, H.; Dreyer, D.; Adden, A.; Brauburger, K.; Heinze, S. The Australian Bogong moth Agrotis infusa: A long-distance nocturnal navigator. Front. Behav. Neurosci. 2016, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.H. Testing Ideas about Australian Insect Migration Using Entomological Radar Observations and a Regional Scale Meteorological Model. Ph.D. Thesis, University of New South Wales, Canberra, Australia, 2016. [Google Scholar]
- Dean, T.J.; Drake, V.A. Monitoring insect migration with radar: The ventral-aspect polarization pattern and its potential for target identification. Int. J. Remote Sens. 2005, 26, 3957–3974. [Google Scholar] [CrossRef]
- Drake, V.A. Distinguishing target classes in observations from vertically pointing entomological radars. Int. J. Remote Sens. 2016, 37, 3811–3835. [Google Scholar] [CrossRef]
- Harman, I.T.; Drake, V.A. Insect monitoring radar: Analytical time-domain algorithm for retrieving trajectory and target parameters. Comput. Electron. Agric. 2004, 43, 23–41. [Google Scholar] [CrossRef]
- Drake, V.A. Automatically operating radars for monitoring insect pest migrations. Insect Sci. 2002, 9, 27–39. [Google Scholar] [CrossRef]
- Drake, V.A.; Chapman, J.W.; Lim, K.S.; Reynolds, D.R.; Riley, J.R.; Smith, A.D. Ventral-aspect radar cross sections and polarization patterns of insects at X band and their relation to size and form. Int. J. Remote Sens. 2017, 38, 5022–5044. [Google Scholar] [CrossRef]
- Hurley, P. TAPM V4. Part. 1: Technical Description; CSIRO Marine and Atmospheric Research: Canberra, Austrilia, 2008. [Google Scholar]
- Hurley, P.; Edwards, M.; Luhar, A.K. TAPM V4. Part. 2: Summary of Some Verification Studies; CSIRO Marine and Atmospheric Research: Canberra, Australia, 2008. [Google Scholar]
- Taylor, J.R.; Zawar-Reza, P.; Low, D.J.; Aryal, P. Verification of a mesoscale model using boundary layer wind profiler data. In Proceedings of the Australian Institute of Physics 16th Biennial Congress, Canberra, Australia, 30 January–4 February 2005. [Google Scholar]
- Gregg, P.C.; Fitt, G.P.; Coombs, M.; Henderson, G.S. Migrating moths (Lepidoptera) collected in tower-mounted light traps in northern New South Wales, Australia: Species composition and seasonal abundance. Bull. Entomol. Res. 1993, 83, 563–578. [Google Scholar] [CrossRef]
- Drake, V.A.; Helm, K.F.; Readshaw, J.L.; Reid, D.G. Insect migration across Bass Strait during spring: A radar study. Bull. Entomol. Res. 1981, 71, 449–466. [Google Scholar] [CrossRef]
- McDonald, G.; Bryceson, K.P.; Farrow, R.A. The development of the 1983 outbreak of the common armyworm, Mythimna convecta, in eastern Australia. J. Appl. Ecol. 1990, 27, 1001–1019. [Google Scholar] [CrossRef]
- Rennie, S.J. Common orientation and layering of migrating insects in southeastern Australia observed with a Doppler weather radar. Meteorol. Appl. 2013, 21, 218–229. [Google Scholar] [CrossRef]
- Deveson, E.D. Satellite normalized difference vegetation index data used in managing Australian plague locusts. J. Appl. Remote Sens. 2013, 7, 075096. [Google Scholar] [CrossRef] [Green Version]
- Drake, V.A.; Farrow, R.A. The nocturnal migration of the Australian plague locust, Chortoicetes terminifera (Walker) (Orthoptera, Acrididae): Quantitative radar observations of a series of northward flights. Bull. Entomol. Res. 1983, 73, 567–585. [Google Scholar] [CrossRef]
- Farrow, R.A.; McDonald, G. Migration strategies and outbreaks of noctuid pests in Australia. Insect Sci. Appl. 1987, 8, 531–542. [Google Scholar] [CrossRef]
- Fitt, G.P.; Dillon, M.L.; Hamilton, J.G. Spatial dynamics of Helicoverpa populations in Australia: Simulation modelling and empirical studies of adult movement. Comput. Electron. Agric. 1995, 13, 177–192. [Google Scholar] [CrossRef]
- Common, I. Migration and gregarious aestivation in the Bogong moth, Agrotis infusa. Nature 1952, 170, 981. [Google Scholar] [CrossRef]
- Gibson, R.K.; Broome, L.; Hutchinson, M.F. Susceptibility to climate change via effects on food resources: The feeding ecology of the endangered mountain pygmy-possum (Burramys parvus). Wildl. Res. 2018, 45. [Google Scholar] [CrossRef]
- Greenup, L. Ecological Studies on the Common Armyworm: Pseudaletia convecta Walk. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 1970. [Google Scholar]
- Mirkovic, D.; Stepanian, P.M.; Wainwright, C.E.; Reynolds, D.R.; Menz, M.H.M. Characterizing animal anatomy and internal composition for electromagnetic modelling in radar entomology. Remote Sens. Ecol. Conserv. 2019, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.W.; Yang, F.; Li, P.; Liu, X.D.; Wu, Q.L.; Hu, G.; Zhai, B.P. Female bias in an immigratory population of Cnaphalocrocis medinalis moths based on field surveys and laboratory tests. Sci. Rep. 2019, 9, 18388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, J.W.; Reynolds, D.R.; Mouritsen, H.; Hill, J.K.; Riley, J.R.; Sivell, D.; Smith, A.D.; Woiwod, I.P. Wind selection and drift compensation optimize migratory pathways in a high-flying moth. Curr. Biol. 2008, 18, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Dean, T.J. Development and Evaluation of Automated Radar Systems for Monitoring and Characterising Echoes from Insect Targets. Ph.D. Thesis, University of New South Wales, Canberra, Australia, 2007. [Google Scholar]







| Jul | Aug | Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004–2005 | – | – | – | – | 6 | 29 | 31 | 27 | 30 | 29 | 18 | 20 |
| 2005–2006 | 27 | 28 | 30 | 31 | 30 | 30 | 30 | 27 | 28 | 20 | 31 | 27 |
| 2006–2007 | 31 | 31 | 30 | 30 | 30 | 31 | 27 | 27 | 30 | 30 | 25 | 30 |
| 2007–2008 | 31 | 31 | 30 | 31 | 5 | – | 11 | 28 | 31 | 30 | 31 | 30 |
| 2008–2009 | 31 | 31 | 30 | 31 | 30 | 20 | – | – | – | – | – | – |
| 2009–2010 | – | – | – | – | – | – | 23 | 28 | 31 | 30 | 31 | 30 |
| 2010–2011 | 31 | 31 | 29 | 31 | 30 | 29 | 31 | 25 | 27 | 30 | 25 | 29 |
| 2011–2012 | 30 | 28 | 21 | 23 | 26 | 28 | 31 | 28 | 31 | 29 | 30 | 29 |
| 2012–2013 | 30 | 30 | 29 | 30 | 29 | 29 | – | – | – | – | – | – |
| 2013–2014 | – | – | 14 | 31 | 28 | 29 | 25 | 27 | 31 | 27 | 31 | – |
| 2014–2015 | – | – | 19 | 29 | 21 | 28 | 28 | 27 | 28 | 27 | 28 | – |
| 2015–2016 | – | – | 26 | 31 | 30 | 31 | 31 | 27 | 29 | 29 | 31 | – |
| 2016–2017 | – | – | 27 | 31 | 30 | 29 | 27 | 27 | 30 | 30 | 30 | – |
| Total | 213 | 210 | 285 | 329 | 295 | 316 | 293 | 298 | 326 | 311 | 311 | 195 |
| a0 (in dBsc) | p | q | fw (Hz) | Mass Range (mg) 2 | |
|---|---|---|---|---|---|
| Class A | −3.3, 6.7 | 0.58, 0.83 | 0.73, 1.28 | 22.6, 35 | 143–395 |
| Classes B and D 3 | −3.6, 4.9 | 0.88, 1 | 0.82, 1.25 | 21, 29 | 105–245 119–282 |
| Class C | −3.3, 4 | 0.9, 1 | 1.27, 1.6 | 21, 28.2 | 121–258 |
| Cluster A | Cluster B | Cluster C | Cluster D | Cluster E | |
|---|---|---|---|---|---|
| fw (mean ± std) 1 | 27.9 ± 3.7 | 31.5 ± 6.7 | 26.1 ± 4.4 | 27.7 ± 4.8 | 31.7 ± 6.6 |
| T (mean ± std) 2 | 24.2 ± 3.8 | 19.8 ± 5.5 | 23.9 ± 3.7 | 16.0 ± 4.7 | 21.2 ± 5.4 |
| coefficient estimate | 1.134 ± 0.001 | 1.496 ± 0.002 | 1.076 ± 0.002 | 1.610 ± 0.015 | 1.413 ± 0.002 |
| Min (mg) | Max (mg) | Mean (mg) | Range 2 (mg) | a0 (dBsc) | Point Lookout | Mt Dowe | Trangie | Stanley | |
|---|---|---|---|---|---|---|---|---|---|
| Austracris guttulosa (N = 20) | 728 | 2391 | 1375 | 880–1869 | 4.7–8.1 | ||||
| Chortoicetes terminifera (N = 24) | 244 | 860 | 415 | 241–589 | 0.5–4.2 | ||||
| Agrotis infusa (N = 54) | 163 | 382 | 231 | 189–273 | (−0.6)–1.1 | 20.4% | 94.3% | 10% | ~10% |
| Agrotis munda (N = 3) | 124 | 183 | 146 | - | (−2.9)–(−0.8) | 1.8% | 0.8% | 15% | ~40% |
| Helicoverpa armigera (N = 4) | 90 | 197 | 141 | - | (−5.1)–(−4.1) | 4.4% | 0.1% | ||
| Mythimna convecta | wingspan is about 40 mm while A. infusa has an average of 47mm | 18.5% | 0.4% | ||||||
| Helicoverpa punctigera (N = 22) | 40 | 152 | 106 | 77–133 | (−6.4)–(−2.5) | 6.1% | 1.5% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Drake, V.A.; Taylor, J.R.; Warrant, E. Insect Target Classes Discerned from Entomological Radar Data. Remote Sens. 2020, 12, 673. https://doi.org/10.3390/rs12040673
Hao Z, Drake VA, Taylor JR, Warrant E. Insect Target Classes Discerned from Entomological Radar Data. Remote Sensing. 2020; 12(4):673. https://doi.org/10.3390/rs12040673
Chicago/Turabian StyleHao, Zhenhua, V. Alistair Drake, John R. Taylor, and Eric Warrant. 2020. "Insect Target Classes Discerned from Entomological Radar Data" Remote Sensing 12, no. 4: 673. https://doi.org/10.3390/rs12040673