Using 1st Derivative Reflectance Signatures within a Remote Sensing Framework to Identify Macroalgae in Marine Environments
Abstract
:1. Introduction
- Ascertain the reflectance signature of species within the functional macroalgae groups found during sampling at the site of interest.
- Quantify the differences in spectral reflectance profiles between sampled macroalgae groups.
- Identify and discriminate between sampled macroalgae groups based on the results from (1) and (2).
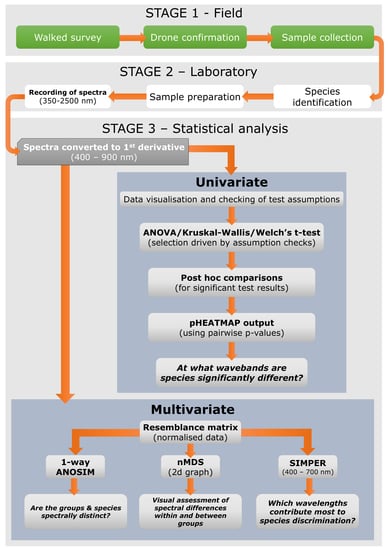
2. Materials and Methods
2.1. Site Selection
2.2. Data Collection
2.3. Laboratory Sampling of Spectral Reflectance
2.4. Data Analysis
2.4.1. Inter-Specific Spectral Differences
2.4.2. Formal Testing of Spectral Differences between Groups (and Species) with ANOSIM
2.4.3. Wavelength Analysis to Find the Best Discriminating Wavelengths (SIMPER)
3. Results
3.1. Laboratory Sampling of Spectral Reflectance
3.2. Data Analysis
3.2.1. Inter-Specific Spectral Differences
3.2.2. Formal Testing of Spectral Differences between Groups (and Species) with ANOSIM
3.2.3. Wavelength Analysis to Find the Best Discriminating Wavelengths (SIMPER)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Lapointe, B.E.; Bedford, B.J. Drift rhodophyte blooms emerge in Lee County, Florida, USA: Evidence of escalating coastal eutrophication. Harmful Algae 2007, 6, 421–437. [Google Scholar] [CrossRef]
- Brand, L.E.; Compton, A. Long-term increase in Karenia brevis abundance along the Southwest Florida Coast. Harmful Algae 2007, 6, 232–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, R.L. The Occurrence of “Green Tides”—A Review; Springer: Berlin, Germany, 1996; pp. 7–43. [Google Scholar] [CrossRef]
- Ye, N.H.; Zhang, X.W.; Mao, Y.Z.; Liang, C.W.; Xu, D.; Zou, J.; Zhuang, Z.M.; Wang, Q.Y. ‘Green tides’ are overwhelming the coastline of our blue planet: Taking the world’s largest example. Ecol. Res. 2011, 26, 477–485. [Google Scholar] [CrossRef]
- Glibert, P.; Seitzinger, S.; Heil, C.; Burkholder, J.; Parrow, M.; Codispoti, L.; Kelly, V. The Role of Eutrophication in the Global Proliferation of Harmful Algal Blooms. Oceanography 2005, 18, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Howarth, R.W.; Sharpley, A.; Walker, D. Sources of nutrient pollution to coastal waters in the United States: Implications for achieving coastal water quality goals. Estuaries 2002, 25, 656–676. [Google Scholar] [CrossRef]
- Pang, S.J.; Liu, F.; Shan, T.F.; Xu, N.; Zhang, Z.H.; Gao, S.Q.; Chopin, T.; Sun, S. Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar. Environ. Res. 2010, 69, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [Green Version]
- De Vries, I.; Philippart, C.J.M.; DeGroodt, E.G.; van der Tol, M.W.M. Coastal Eutrophication and Marine Benthic Vegetation: A Model Analysis; Springer: Berlin/Heidelberg, Germany, 1996; pp. 79–113. [Google Scholar] [CrossRef]
- Shen, H.; Perrie, W.; Liu, Q.; He, Y. Detection of macroalgae blooms by complex SAR imagery. Mar. Pollut. Bull. 2014, 78, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Villacorte, L.; Tabatabai, S.; Dhakal, N.; Amy, G.; Schippers, J.; Kennedy, M. Algal blooms: An emerging threat to seawater reverse osmosis desalination. Desalin. Water Treat. 2015, 55, 2601–2611. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Zingone, A.; Oksfeldt Enevoldsen, H. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-Bonaca, M. Trace elements in Mediterranean seagrasses and macroalgae. A review. Sci. Total Environ. 2018, 618, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Dekker, A.G.; Phinn, S.R.; Anstee, J.; Bissett, P.; Brando, V.E.; Casey, B.; Fearns, P.; Hedley, J.; Klonowski, W.; Lee, Z.P.; et al. Intercomparison of Shallow Water Bathymetry, Hydro-Optics, and Benthos Mapping Techniques in Australian and Caribbean Coastal Environments. Limnol. Oceanogr. 2011, 9, 396–425. [Google Scholar] [CrossRef]
- Hedley, J.; Enríquez, S. Optical properties of canopies of the tropical seagrass Thalassia testudinum estimated by a three-dimensional radiative transfer model. Limnol. Oceanogr. 2010. [Google Scholar] [CrossRef]
- Hedley, J.; Russell, B.; Randolph, K.; Dierssen, H. A physics-based method for the remote sensing of seagrasses. Remote Sens. Environ. 2016, 174, 134–147. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Zimmerman, R.C.; Leathers, R.A.; Downes, T.V.; Davis, C.O. Ocean color remote sensing of seagrass and bathymetry in the Bahamas Banks by high-resolution airborne imagery. Limnol. Oceanogr. 2003, 48, 444–455. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Feng, L.; Hardy, R.F. Spectral and spatial requirements of remote measurements of pelagic Sargassum macroalgae. Remote Sens. Environ. 2015, 167, 229–246. [Google Scholar] [CrossRef]
- Parsiani, H.; Torres, M.; Rodriguez, P.A. High-resolution vegetation index as measured by radar and its validation with spectrometer. In Proceedings of the Image and Signal Processing for Remote Sensing X, Canary Islands, Spain, 13–16 September 2004; p. 284. [Google Scholar] [CrossRef]
- Zhang, X.; Kondragunta, S.; Ram, J.; Schmidt, C.; Huang, H.C.; Zhang, X. Near-real-time global biomass burning emissions product from geostationary satellite constellation. J. Geophys. Res. 2012, 117, D14. [Google Scholar] [CrossRef]
- Saunders, C.; Bird, R.; Da Silva, A.; Sweeting, M.; Gomes, L. Design considerations in rapid-revisit small satellite constellations. In Proceedings of the 68th International Astronautical Congress: Unlocking Imagination, Fostering Innovation and Strengthening Security, Adelaide, Australia, 25–29 September 2017; pp. 5961–5984. [Google Scholar]
- Lubac, B.; Loisel, H. Variability and classification of remote sensing reflectance spectra in the eastern English Channel and southern North Sea. Remote Sens. Environ. 2007, 110, 45–58. [Google Scholar] [CrossRef]
- Zou, W.; Yuan, L.; Zhang, L. Analyzing the spectral response of submerged aquatic vegetation in a eutrophic lake, Shanghai, China. Ecol. Eng. 2013, 57, 65–71. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.; Gao, X.; Ferreira, L. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Latifi, H.; Stereńczak, K.; Modzelewska, A.; Lefsky, M.; Waser, L.T.; Straub, C.; Ghosh, A. Review of studies on tree species classification from remotely sensed data. Remote Sens. Environ. 2016, 186, 64–87. [Google Scholar] [CrossRef]
- Längkvist, M.; Kiselev, A.; Alirezaie, M.; Loutfi, A. Classification and Segmentation of Satellite Orthoimagery Using Convolutional Neural Networks. Remote Sens. 2016, 8, 329. [Google Scholar] [CrossRef]
- Pérez-Ortiz, M.; Peña, J.M.; Gutiérrez, P.A.; Torres-Sánchez, J.; Hervás-Martínez, C.; López-Granados, F. Selecting patterns and features for between- and within- crop-row weed mapping using UAV-imagery. Expert Syst. Appl. 2016, 47, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Tin, H.C.; O’Leary, M.; Fotedar, R.; Garcia, R. Spectral response of marine submerged aquatic vegetation: A case study in Western Australia coast. In Proceedings of the OCEANS 2015—MTS/IEEE Washington, Washington, DC, USA, 19–22 October 2015; pp. 1–5. [Google Scholar] [CrossRef]
- Fyfe, S.K. Spatial and temporal variation in spectral reflectance: Are seagrass species spectrally distinct? Limnol. Oceanogr. 2003, 48, 464–479. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Hu, C.; Ming-Xia, H. Remote estimation of biomass of Ulva prolifera macroalgae in the Yellow Sea. Remote Sens. Environ. 2017, 192, 217–227. [Google Scholar] [CrossRef]
- Xing, Q.; Hu, C. Mapping macroalgal blooms in the Yellow Sea and East China Sea using HJ-1 and Landsat data: Application of a virtual baseline reflectance height technique. Remote Sens. Environ. 2016, 178, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Fang, J.; Zhang, J.; Ren, L.; Mao, Y.; Li, B.; Zhang, M.; Liu, D.; Du, M. The impact of the herbicide atrazine on growth and photosynthesis of seagrass, Zostera marina (L.), seedlings. Mar. Pollut. Bull. 2011, 62, 1628–1631. [Google Scholar] [CrossRef]
- Simic, A.; Chen, J.M. Refining a hyperspectral and multiangle measurement concept for vegetation structure assessment. Can. J. Remote Sens. 2008, 34, 174–191. [Google Scholar] [CrossRef]
- Nuclear Energy Institute. Economic Impacts of The R.E. Ginna Nuclear Power Plant an Analysis; Technical Report; Nuclear Energy Institute: Washington, DC, USA, 2015. [Google Scholar]
- Labsphere. Spectralon® Diffuse Reflectance Standards; Labsphere|Internationally Recognized Photonics Company: North Sutton, NH, USA, 2018. [Google Scholar]
- Danner, M.; Locherer, M.; Hank, T.; Richter, K. Spectral Sampling with the ASD FieldSpec 4; GFZ Data Services: Potsdam, Germany, 2015. [Google Scholar] [CrossRef]
- Fabre, S.; Lesaignoux, A.; Olioso, A.; Briottet, X. Influence of Water Content on Spectral Reflectance of Leaves in the 3–15 μm Domain. IEEE Geosci. Remote Sens. Lett. 2011, 8, 143–147. [Google Scholar] [CrossRef]
- Garcia, R.; Hedley, J.; Tin, H.; Fearns, P.; Garcia, R.A.; Hedley, J.D.; Tin, H.C.; Fearns, P.R.C.S. A Method to Analyze the Potential of Optical Remote Sensing for Benthic Habitat Mapping. Remote Sens. 2015, 7, 13157–13189. [Google Scholar] [CrossRef] [Green Version]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994; p. 509. [Google Scholar]
- ASD. Indico Pro; Malvern Panalytical: Malvern, UK, 2017. [Google Scholar]
- Hong, Y.; Liu, Y.; Chen, Y.; Liu, Y.; Yu, L.; Liu, Y.; Cheng, H. Application of fractional-order derivative in the quantitative estimation of soil organic matter content through visible and near-infrared spectroscopy. Geoderma 2019, 337, 758–769. [Google Scholar] [CrossRef]
- Fligner, M.A.; Killeen, T.J. Distribution-Free Two-Sample Tests for Scale. J. Am. Stat. Assoc. 1976, 71, 210–213. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Bartlett, M.S. A Note on the Multiplying Factors for Various χ2 Approximations. J. R. Stat. Soc. 1954. [Google Scholar] [CrossRef]
- Delacre, M.; Lakens, D.; Leys, C. Why Psychologists Should by Default Use Welch’s t-test Instead of Student’s t-test. Int. Rev. Soc. Psychol. 2017, 30, 92. [Google Scholar] [CrossRef]
- Jaccard, J.; Becker, M.A.; Wood, G. Pairwise multiple comparison procedures: A review. Psychol. Bull. 1984, 96, 589–596. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps. 2018. Available online: https://cran.mtu.edu/web/packages/pheatmap/index.html (accessed on 28 January 2019).
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.r-project.org/ (accessed on 28 January 2019).
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Michin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 28 January 2019).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Clarke, R.; Gorley, R. PRIMER-E. 2015. Available online: http://www.primer-e.com/ (accessed on 28 January 2019).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Buttigieg, P.L.; Ramette, A. A guide to statistical analysis in microbial ecology: A community-focused, living review of multivariate data analyses. FEMS Microbiol. Ecol. 2014, 90, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Baeck, P.; Blommaert, J.; Delalieux, S.; Delauré, B.; Livens, S.; Nuyts, D.; Sima, A.; Jacquemin, G.; Goffart, J.P.; Nv, V.; et al. High resolution vegetation mapping with a novel compact hyperspectral camera system. In Proceedings of the 13th International Conference on Precision Agriculture, St. Louis, MO, USA, 31 July–4 August 2016. [Google Scholar]
- Wells, E. A Field Guide to the British Seaweeds as Required for Assistance in the Classification of Water Bodies under the Water Framework Directive; Environment Agency: Bristol, UK, 1997. [Google Scholar]
- Vaughan, A. In a Laver: Seaweed Shuts Nuclear Reactor Again in Bad Weather. The Guardian, 5 March 2018. [Google Scholar]
- Matsumura, K.; Kamiya, K.; Yamashita, K.; Hayashi, F.; Watanabe, I.; Murao, Y.; Miyasaka, H.; Kamimura, N.; Nogami, M. Genetic polymorphism of the adult medusae invading an electric power station and wild polyps of Aurelia aurita in Wakasa Bay, Japan. J. Mar. Biol. Assoc. UK 2005, 85, 563–568. [Google Scholar] [CrossRef]
- Barath Kumar, S.; Mohanty, A.; Das, N.; Satpathy, K.; Sarkar, S. Impingement of marine organisms in a tropical atomic power plant cooling water system. Mar. Pollut. Bull. 2017, 124, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Dekker, A.G.; Malthus, T.J.; Wijnen, M.M.; Seyhan, E. Remote sensing as a tool for assessing water quality in Loosdrecht lakes. Hydrobiologia 1992, 233, 137–159. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Clothiaux, E.E.; Ackerman, T.P.; Mace, G.G.; Moran, K.P.; Marchand, R.T.; Miller, M.A.; Martner, B.E. Objective Determination of Cloud Heights and Radar Reflectivities Using a Combination of Active Remote Sensors at the ARM CART Sites. J. Appl. Meteorol. 2000, 39, 645–665. [Google Scholar] [CrossRef]
- Suwandana, E.; Kawamura, K.; Sakuno, Y.; Evri, M.; Lesmana, A.H. Hyperspectral Reflectance Response of Seagrass (Enhalus acoroides) and Brown Algae (Sargassum sp.) to Nutrient Enrichment at Laboratory Scale. J. Coast. Res. 2012, 283, 956–963. [Google Scholar] [CrossRef]
- He, K.S.; Rocchini, D.; Neteler, M.; Nagendra, H. Benefits of hyperspectral remote sensing for tracking plant invasions. Divers. Distrib. 2011, 17, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Carrasco-Escobar, G.; Manrique, E.; Ruiz-Cabrejos, J.; Saavedra, M.; Alava, F.; Bickersmith, S.; Prussing, C.; Vinetz, J.M.; Conn, J.E.; Moreno, M.; et al. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl. Trop. Dis. 2019, 13, e0007105. [Google Scholar] [CrossRef]
- Jay, S.; Baret, F.; Dutartre, D.; Malatesta, G.; Héno, S.; Comar, A.; Weiss, M.; Maupas, F. Exploiting the centimeter resolution of UAV multispectral imagery to improve remote-sensing estimates of canopy structure and biochemistry in sugar beet crops. Remote Sens. Environ. 2018. [Google Scholar] [CrossRef]
- Atzberger, C.; Eilers, P.H.C. Evaluating the effectiveness of smoothing algorithms in the absence of ground reference measurements. Int. J. Remote Sens. 2011, 32, 3689–3709. [Google Scholar] [CrossRef]
- Atzberger, C.; Eilers, P.H. A time series for monitoring vegetation activity and phenology at 10-daily time steps covering large parts of South America. Int. J. Digit. Earth 2011, 4, 365–386. [Google Scholar] [CrossRef]
- Atzberger, C.; Wess, M.; Doneus, M.; Verhoeven, G. ARCTIS—A MATLAB® Toolbox for Archaeological Imaging Spectroscopy. Remote Sens. 2014, 6, 8617–8638. [Google Scholar] [CrossRef]
- Meroni, M.; Fasbender, D.; Rembold, F.; Atzberger, C.; Klisch, A. Near real-time vegetation anomaly detection with MODIS NDVI: Timeliness vs. accuracy and effect of anomaly computation options. Remote Sens. Environ. 2019, 221, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Atzberger, C.; Klisch, A.; Mattiuzzi, M.; Vuolo, F.; Atzberger, C.; Klisch, A.; Mattiuzzi, M.; Vuolo, F. Phenological Metrics Derived over the European Continent from NDVI3g Data and MODIS Time Series. Remote Sens. 2013, 6, 257–284. [Google Scholar] [CrossRef] [Green Version]
- Doneus, M.; Verhoeven, G.; Atzberger, C.; Wess, M.; Ruš, M. New ways to extract archaeological information from hyperspectral pixels. J. Archaeol. Sci. 2014, 52, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Rembold, F.; Atzberger, C.; Savin, I.; Rojas, O.; Rembold, F.; Atzberger, C.; Savin, I.; Rojas, O. Using Low Resolution Satellite Imagery for Yield Prediction and Yield Anomaly Detection. Remote Sens. 2013, 5, 1704–1733. [Google Scholar] [CrossRef] [Green Version]
- Zacharias, M.; Niemann, O.; Borstad, G. An Assessment and Classification of a Multispectral Bandset for the Remote Sensing of Intertidal Seaweeds. Can. J. Remote Sens. 1992, 18, 263–274. [Google Scholar] [CrossRef]
- Gong, P.; Pu, R.; Yu, B. Conifer species recognition: An exploratory analysis of In Situ Hyperspectral data. Remote Sens. Environ. 1997, 62, 189–200. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration; Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001. [Google Scholar] [CrossRef]
- Wernberg, T.; Krumhansl, K.; Filbee-Dexter, K.; Pedersen, M.F. Status and Trends for the World’s Kelp Forests. In World Seas: An Environmental Evaluation; Academic Press: Cambridge, MA, USA, 2019; pp. 57–78. [Google Scholar] [CrossRef]
- Sridhar, B.B.M.; Han, F.X.; Diehl, S.V.; Monts, D.L.; Su, Y. Spectral reflectance and leaf internal structure changes of barley plants due to phytoextraction of zinc and cadmium. Int. J. Remote Sens. 2007, 28, 1041–1054. [Google Scholar] [CrossRef]
- Pacumbaba, R.; Beyl, C. Changes in hyperspectral reflectance signatures of lettuce leaves in response to macronutrient deficiencies. Adv. Space Res. 2011, 48, 32–42. [Google Scholar] [CrossRef]
- Datt, B. Recognition of Eucalyptus forest species using hyperspectral reflectance data. In Proceedings of the IEEE 2000 International Geoscience and Remote Sensing Symposium. Taking the Pulse of the Planet: The·Role of Remote Sensing in Managing the Environment, Honolulu, HI, USA, 24–28 July 2000; Volume 4, pp. 1405–1407. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Schmidt, K.; Skidmore, A. Spectral discrimination of vegetation types in a coastal wetland. Remote Sens. Environ. 2003, 85, 92–108. [Google Scholar] [CrossRef]
- Morris, W.D.; Witte, W.G.; Whitlock, C.H. Turbid Water Measurements of Remote Sensing Penetration Depth at Visible and Near-Infrared Wavelength; Technical Report; NASA—Langley Research Center: Hampton, VA, USA, 1980. [Google Scholar]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.; Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative Remote Sensing at Ultra-High Resolution with UAV Spectroscopy: A Review of Sensor Technology, Measurement Procedures, and Data Correction Workflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- Greenwood, W.W.; Lynch, J.P.; Zekkos, D. Applications of UAVs in Civil Infrastructure. J. Infrastruct. Syst. 2019, 25, 04019002. [Google Scholar] [CrossRef]
- Gray, P.; Ridge, J.; Poulin, S.; Seymour, A.; Schwantes, A.; Swenson, J.; Johnston, D.; Gray, P.C.; Ridge, J.T.; Poulin, S.K.; et al. Integrating Drone Imagery into High Resolution Satellite Remote Sensing Assessments of Estuarine Environments. Remote Sens. 2018, 10, 1257. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; Mclean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: Surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote Sens. 2019, 40, 407–419. [Google Scholar] [CrossRef]
- Yang, C. A high-resolution airborne four-camera imaging system for agricultural remote sensing. Comput. Electron. Agric. 2012, 88, 13–24. [Google Scholar] [CrossRef]
- Tang, L.; Shao, G. Drone remote sensing for forestry research and practices. J. For. Res. 2015, 26, 791–797. [Google Scholar] [CrossRef]
- Singh, K.D.; Nansen, C. Advanced calibration to improve robustness of drone-acquired hyperspectral remote sensing data. In Proceedings of the 2017 6th International Conference on Agro-Geoinformatics, Fairfax, VA, USA, 7–10 August 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Bostater, C.R.; Jones, J.; Frystacky, H.; Kovacs, M.; Jozsa, O. Integration, testing, and calibration of imaging systems for land and water remote sensing. In Proceedings of the Remote Sensing of the Ocean, Sea Ice, and LargeWater Regions 2010, Toulouse, France, 20–23 September 2010; Volume 7825, p. 78250N. [Google Scholar] [CrossRef]
- Larson, M.D.; Simic Milas, A.; Vincent, R.K.; Evans, J.E. Multi-depth suspended sediment estimation using high-resolution remote-sensing UAV in Maumee River, Ohio. Int. J. Remote Sens. 2018, 39, 5472–5489. [Google Scholar] [CrossRef]






| Kelp | Fucoids | Other |
|---|---|---|
| Laminaria saccharina (n = 230) | Fucus vesiculosus (n = 290) | Ulva lactuca (n = 381) |
| Laminaria sp. (n = 1177) | Fucus serratus (n = 612) | |
| Laminaria sp. stipe (n = 68) | Fucus spiralis (n = 228) | |
| Alaria esculenta (n = 47) |
| Kelp | Fucoid | Other | |
|---|---|---|---|
| Kelp | - | - | - |
| Fucoid | 0.539 | - | - |
| Other | 0.455 | 0.712 | - |
| Alaria esculenta | Fucus serratus | Fucus spiralis | Fucus vesiculosus | Laminaria saccharina | Laminaria sp. | Laminaria sp. stipe | Ulva lactuca | |
|---|---|---|---|---|---|---|---|---|
| Alaria esculenta | - | - | - | - | - | - | - | - |
| Fucus serratus | 0.885 | - | - | - | - | - | - | - |
| Fucus spiralis | 0.750 | 0.331 | - | - | - | - | - | - |
| Fucus vesiculosis | 0.631 | 0.457 | 0.140 | - | - | - | - | - |
| Laminaria saccharina | 0.451 | 0.930 | 0.824 | 0.707 | - | - | - | - |
| Laminariasp. | 0.0617 (0.092) | 0.583 | 0.610 | 0.492 | 0.159 | - | - | - |
| Laminariasp. stipe | 0.795 | 0.969 | 0.786 | 0.559 | 0.872 | 0.685 | - | - |
| Ulva lactuca | 0.490 | 0.848 | 0.830 | 0.805 | 0.797 | 0.533 | 0.939 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mcilwaine, B.; Casado, M.R.; Leinster, P. Using 1st Derivative Reflectance Signatures within a Remote Sensing Framework to Identify Macroalgae in Marine Environments. Remote Sens. 2019, 11, 704. https://doi.org/10.3390/rs11060704
Mcilwaine B, Casado MR, Leinster P. Using 1st Derivative Reflectance Signatures within a Remote Sensing Framework to Identify Macroalgae in Marine Environments. Remote Sensing. 2019; 11(6):704. https://doi.org/10.3390/rs11060704
Chicago/Turabian StyleMcilwaine, Ben, Monica Rivas Casado, and Paul Leinster. 2019. "Using 1st Derivative Reflectance Signatures within a Remote Sensing Framework to Identify Macroalgae in Marine Environments" Remote Sensing 11, no. 6: 704. https://doi.org/10.3390/rs11060704