Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of MXene
2.3. Electrochemical Procedures
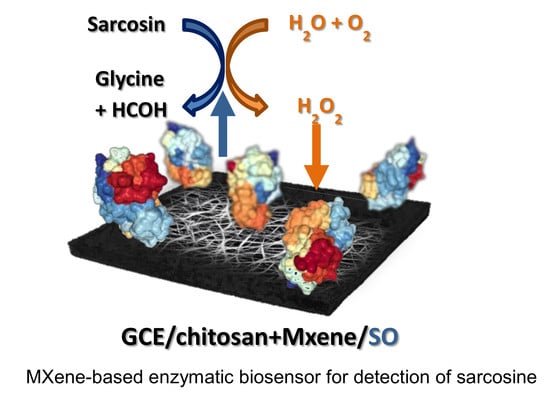
2.4. Construction of MXene-Based Sarcosine Biosensor
2.5. Contact Angle Measurements
2.6. Characterisation of MXene and MXene-Chitosan Composite
3. Results and Discussion
3.1. Characterisation of MXene
3.2. Electrochemical Measurements
3.3. Clinical Application of SOx/MXene-chi/GCE Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tkac, J.; Gajdosova, V.; Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Kasak, P. Prostate-specific antigen glycoprofiling as diagnostic and prognostic biomarker of prostate cancer. Interface Focus. 2019, 9, 20180077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertok, T.; Lorencova, L.; Hroncekova, S.; Gajdosova, V.; Jane, E.; Hires, M.; Kasak, P.; Kaman, O.; Sokol, R.; Bella, V.; et al. Advanced impedimetric biosensor configuration and assay protocol for glycoprofiling of a prostate oncomarker using Au nanoshells with a magnetic core. Biosens. Bioelectron. 2019, 131, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Damborský, P.; Damborská, D.; Belický, Š.; Tkáč, J.; Katrlík, J. Sweet Strategies in Prostate Cancer Biomarker Research: Focus on a Prostate Specific Antigen. BioNanoScience 2017, 8, 690–700. [Google Scholar] [CrossRef]
- Markin, P.A.; Brito, A.; Moskaleva, N.; Fodor, M.; Lartsova, E.V.; Shpot, Y.V.; Lerner, Y.V.; Mikhajlov, V.Y.; Potoldykova, N.V.; Enikeev, D.V. Plasma Sarcosine Measured by Gas Chromatography-Mass Spectrometry Distinguishes Prostatic Intraepithelial Neoplasia and Prostate Cancer from Benign Prostate Hyperplasia. Lab. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mazzu-Nascimento, T.; Gomes Carneiro Leão, P.A.; Catai, J.R.; Morbioli, G.G.; Carrilho, E. Towards low-cost bioanalytical tools for sarcosine assays for cancer diagnostics. Anal. Methods 2016, 8, 7312–7318. [Google Scholar] [CrossRef]
- Burton, C.; Gamagedara, S.; Ma, Y. A novel enzymatic technique for determination of sarcosine in urine samples. Anal. Methods 2012, 4, 141–146. [Google Scholar] [CrossRef]
- Cernei, N.; Zitka, O.; Ryvolova, M.; Adam, V.; Masarik, M.; Hubalek, J.; Kizek, R. Spectrometric and Electrochemical Analysis of Sarcosine as a Potential Prostate Carcinoma Marker. Int. J. Electrochem. Sci. 2012, 7, 4286–4301. [Google Scholar]
- Jiang, Y.; Cheng, X.; Wang, C.; Ma, Y. Quantitative Determination of Sarcosine and Related Compounds in Urinary Samples by Liquid Chromatography with Tandem Mass Spectrometry. Anal. Chem. 2010, 82, 9022–9027. [Google Scholar] [CrossRef]
- Narwal, V.; Kumar, P.; Joon, P.; Pundir, C.S. Fabrication of an amperometric sarcosine biosensor based on sarcosine oxidase/chitosan/CuNPs/c-MWCNT/Au electrode for detection of prostate cancer. Enzyme Microb. Technol. 2018, 113, 44–51. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Naguib, M.; Gogotsi, Y. Synthesis of two-dimensional materials by selective extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3 AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsoum, M.W. The MN+1AXN Phases: A New Class of Solids; Thermodynamically Stable Nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 9921005. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Dosekova, E.; Holazova, A.; Paprckova, D.; Vikartovska, A.; Sasinkova, V.; Filip, J.; Kasak, P.; Jerigova, M.; et al. Electrochemical performance of Ti3C2Tx MXene in aqueous media: Towards ultrasensitive H2O2 sensing. Electrochim. Acta 2017, 235, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorencova, L.; Bertok, T.; Filip, J.; Jerigova, M.; Velic, D.; Kasak, P.; Mahmoud, K.A.; Tkac, J. Highly stable Ti3C2Tx (MXene)/Pt nanoparticles-modified glassy carbon electrode for H2O2 and small molecules sensing applications. Sens. Actuat. B. Chem. 2018, 263, 360–368. [Google Scholar] [CrossRef]
- Lorencova, L.; Gajdosova, V.; Hroncekova, S.; Bertok, T.; Blahutova, J.; Vikartovska, A.; Parrakova, L.; Gemeiner, P.; Kasak, P.; Tkac, J. 2D MXenes as Perspective Immobilization Platforms for Design of Electrochemical Nanobiosensors. Electroanalysis 2019, 31, 1833–1844. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-Derived Sodium/Potassium Titanate Nanoribbons for High-Performance Sodium/Potassium Ion Batteries with Enhanced Capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Chen, S.; Ding, W.; Nie, Y.; Wei, Z. An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti3C2X2 (X = OH, F) nanosheets for oxygen reduction reaction. Chem. Commun. 2013, 49, 10112–10114. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An Organ-Like Titanium Carbide Material (MXene) with Multilayer Structure Encapsulating Hemoglobin for a Mediator-Free Biosensor. J. Electrochem. Soc. 2015, 162, B16–B21. [Google Scholar] [CrossRef]
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sens. Actuat. B. Chem. 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhang, X.; Ma, L.; Gao, J.; Jiang, Y. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem. Eng. J. 2017, 128, 243249. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Lu, X.; Wu, Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Henderson, C.J.; Campíns-Falcó, P.; Daly, R.; Hall, E.A.H. Towards sarcosine determination in urine for prostatic carcinoma detection. Sens. Actuat. B: Chem. 2019, 287, 380–389. [Google Scholar]
- Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a potential prostate cancer biomarker—A review. Int. J. Mol. Sci. 2013, 14, 13893–13908. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wei, W.; Ke, S.; Zeng, X.; Lin, P. A novel and sensitive sarcosine biosensor based on organic electrochemical transistor. Electrochim. Acta 2019, 307, 100–106. [Google Scholar] [CrossRef]
- Wagner, M.A.; Trickey, P.; Chen, Z.-w.; Mathews, F.S.; Jorns, M.S. Monomeric Sarcosine Oxidase: 1. Flavin Reactivity and Active Site Binding Determinants. Biochemistry 2000, 39, 8813–8824. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hoshika, H.; Inouye, Y.; Ikuta, S.; Matsuura, K.; Nakamura, S. Purificationand Characterization of Sarcosine oxidase of Bacillus Origin. Chem. Pharm. Bull. 1987, 35, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhang, Q.; Dang, Y.; Shu, G. The Effect of Glutaraldehyde Cross-Linking on the Enzyme Activity of Immobilized &beta-Galactosidase on Chitosan Bead. Advance J. Food Sci. Technol. 2013, 5, 932–935. [Google Scholar]
- Ang, L.F.; Por, L.Y.; Yam, M.F. Development of an amperometric-based glucose biosensor to measure the glucose content of fruit. PLoS ONE 2015, 10, e0111859. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-S.; Pan, Q.-X.; Wang, G.-X. A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide. Sensors 2005, 5, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.G.; Li, N.; Li, Q.; Chen, S.Q.; Wang, H.L.; Yang, H.P. Amperometric sarcosine biosensor based on hollow magnetic Pt-Fe3O4@C nanospheres. Anal. Chim. Acta 2019, 1078, 161–167. [Google Scholar] [CrossRef]
- Kumar, P.; Narwal, V.; Jaiwal, R.; Pundir, C.S. Construction and application of amperometric sarcosine biosensor based on SOxNPs/AuE for determination of prostate cancer. Biosens Bioelectron. 2018, 122, 140–146. [Google Scholar] [CrossRef]
- Henderson, C.J.; Pumford, E.; Seevaratnam, D.J.; Daly, R.; Hall, E.A.H. Gene to diagnostic: Self immobilizing protein for silica microparticle biosensor, modelled with sarcosine oxidase. Biomaterials 2019, 193, 58–70. [Google Scholar] [CrossRef]
- Zhao, S.; Volkner, J.; Riedel, M.; Witte, G.; Yue, Z.; Lisdat, F.; Parak, W.J. Multiplexed Readout of Enzymatic Reactions by Means of Laterally Resolved Illumination of Quantum Dot Electrodes. ACS Appl. Mater. Interf. 2019, 11, 21830–21839. [Google Scholar] [CrossRef]
- Pundir, C.S.; Deswal, R.; Kumar, P. Quantitative analysis of sarcosine with special emphasis on biosensors: A review. Biomarkers 2019, 24, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.; Pereira, C.M.; Sales, M.G.; Noronha, J.P.; Costa-Rodrigues, J.; Silva, F.; Fernandes, M.H. Sarcosine oxidase composite screen-printed electrode for sarcosine determination in biological samples. Anal Chim Acta 2014, 850, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhao, Y.; Yang, Q.; Du, D.; Yang, H.; Lin, Y. Amperometric sarcosine biosensor with strong anti-interference capabilities based on mesoporous organic-inorganic hybrid materials. Biosens. Bioelectron. 2019, 141, 111431. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fu, B.; Chen, J.; Li, K. An electrochemical sarcosine sensor based on biomimetic recognition. Microchim. Acta 2019, 186, 136. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Yang, C.; Zhao, X.; Xie, S.; Ge, Z. Nano Pt@ZIF8 Modified Electrode and Its Application to Detect Sarcosine. J. Electrochem. Soc. 2018, 165, H247–H250. [Google Scholar] [CrossRef]



| Enzyme/Protein | MXene Patterning | Immobilization | Analyte | LOD (nM) | Reference |
|---|---|---|---|---|---|
| haemoglobin (Hb) | MXene | Hb glued via Nf | H2O2 | 20 | [23] |
| haemoglobin (Hb) | MXene | Hb glued via Nf | NO2− | 120 | [24] |
| haemoglobin (Hb) | TiO2 on MXene | Hb glued via Nf | H2O2 | 14 | [22] |
| tyrosinase (Tyr) | MXene | Tyr glued via Chi | phenol | 12 | [27] |
| glucose oxidase | AuNPs on MXene | GOx adsorbed on Nf-AuNP/MXene | glucose | 5900 | [25] |
| SOx | MXene | SOx glued via Chi | sarcosine | 18 | This work |
| Interface | LOD (nM) | Working Range (nM) | Reference |
|---|---|---|---|
| platinum-plated anodised aluminium oxide electrode | 50 | 50–100,000 | [30] |
| Hybrid: Pt nanoparticles with hollow Fe3O4 nanospheres | 430 | 500–60,000 | [37] |
| Modified SPCE | 16 | 10–100 | [42] |
| SOxNPs/AuE | 10 | 100–100,000 | [38] |
| SOx/Pt–Fe3O4@C nanocomposite/GCE | 430 | 500–60,000 | [37] |
| Pt-supported organic/inorganic hybrid mesoporous NPs | 130 | 1000–70,000 | [43] |
| Riboflavin/AuPt-PPy/graphene-chitosan-modified GCE | 680 | 2500–600,000 | [44] |
| nanoPt@porous zeolitic imidazolate framework-8 | 1060 | 5000–30,000 | [45] |
| SOx/MXene-Chi/GCE | 18 | 36–7800 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Vikartovska, A.; Tanvir, A.; Kasak, P.; Tkac, J. Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes 2020, 8, 580. https://doi.org/10.3390/pr8050580
Hroncekova S, Bertok T, Hires M, Jane E, Lorencova L, Vikartovska A, Tanvir A, Kasak P, Tkac J. Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes. 2020; 8(5):580. https://doi.org/10.3390/pr8050580
Chicago/Turabian StyleHroncekova, Stefania, Tomas Bertok, Michal Hires, Eduard Jane, Lenka Lorencova, Alica Vikartovska, Aisha Tanvir, Peter Kasak, and Jan Tkac. 2020. "Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples" Processes 8, no. 5: 580. https://doi.org/10.3390/pr8050580