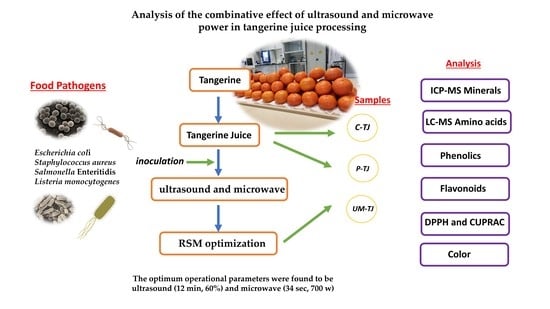
Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining the Tangerine Juice
2.2. Microbial Strain Preparation and Inoculation in Tangerine Juice
2.3. Thermal Pasteurization, Ultrasound, and Microwave Treatments
2.4. Microbial Analysis
2.5. Modeling Inactivation
2.6. Determination of Bioactive Compounds
2.7. Analysis of Amino Acids
2.8. Analysis of Minerals
2.9. Determination of Color Parameters
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimization Conditions Inhibition of Pathogens
3.2. Determination of Bioactive Compounds
3.3. Analysis of Amino Acid
3.4. Analysis of Minerals
3.5. Color Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ertek, N.; Dağdemir, V.; Keskin, A. Marketing Margins and Economic Analysis of the Mandarin Market in Turkey. Atatürk Univ. J. Agric. Fac. 2020, 51, 119–125. [Google Scholar] [CrossRef]
- Kaplan, E.; Taşova, M.; Gülse Bal, H.S. Consumer Preferences According to Fruit Drying Methods: Sunburst Type Mandarin Example. Turkish J. Agric. Eng. Res. 2020, 1, 425–440. [Google Scholar] [CrossRef]
- Kernou, O.N.; Belbahi, A.; Amir, A.; Bedjaoui, K.; Kerdouche, K.; Dairi, S.; Aoun, O.; Madani, K. Effect of sonication on microwave inactivation of Escherichia coli in an orange juice beverage. J. Food Process Eng. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Niveditha, A.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of cold plasma and ozone technology for decontamination of Escherichia coli in foods- a review. Food Control 2021, 130, 108338. [Google Scholar] [CrossRef]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.P.; Martínez De La Peña, C.; Silva, J.L.; Luna-Guevara, M.L. The Role of Pathogenic E. coli in Fresh Vegetables: Behavior, Contamination Factors, and Preventive Measures. Int. J. Microbiol. 2019, 2019, 2894328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2020, 19, 37–54. [Google Scholar] [CrossRef]
- Schneider, G.; Schweitzer, B.; Steinbach, A.; Pertics, B.Z.; Cox, A.; Kőrösi, L. Antimicrobial efficacy and spectrum of phosphorous-fluorine co-doped tio2 nanoparticles on the foodborne pathogenic bacteria campylobacter jejuni, salmonella typhimurium, enterohaemorrhagic e. Coli, yersinia enterocolitica, shewanella putrefaciens, lister. Foods 2021, 10, 1786. [Google Scholar] [CrossRef]
- Cheng, R.M.; Churey, J.J.; Worobo, R.W. Inactivation of Salmonella enterica and spoilage microorganisms in orange juice treated with dimethyl dicarbonate (DMDC). Int. J. Food Microbiol. 2018, 285, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Majidi, M.R.; Khaki, P.; Jahanban-Esfahlan, A.; de la Guardia, M.; Mokhtarzadeh, A. State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1868–1912. [Google Scholar] [CrossRef]
- Guerreiro, D.N.; Pucciarelli, M.G.; Tiensuu, T.; Gudynaite, D.; Boyd, A.; Johansson, J.; García-Del Portillo, F.; O Byrne, C.P. Acid stress signals are integrated into the σB-dependent general stress response pathway via the stressosome in the food-borne pathogen Listeria monocytogenes. PLOS Pathog. 2022, 18, e1010213. [Google Scholar] [CrossRef]
- Parsons, C.; Brown, P.; Kathariou, S. Use of Bacteriophage Amended with CRISPR-Cas Systems to Combat Antimicrobial Resistance in the Bacterial Foodborne Pathogen Listeria monocytogenes. Antibiotics 2021, 10, 308. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Chen, X.; Qu, C. Review controlling Listeria monocytogenes in ready-to-eat meat and poultry products: An overview of outbreaks, current legislations, challenges, and future prospects. Trends Food Sci. Technol. 2021, 116, 24–35. [Google Scholar] [CrossRef]
- Alves, L.; de, L.; dos Santos, R.L.; Bayer, B.L.; Devens, A.L.M.; Cichoski, A.J.; Mendonça, C.R.B. Thermosonication of tangerine juice: Effects on quality characteristics, bioactive compounds, and antioxidant activity. J. Food Process. Preserv. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Rodríguez, M.; Oteiza, J.; Giannuzzi, L.; Zaritzky, N. Evaluation of mutagenicity associated with Escherichia coli inactivation in UV-treated orange juice. Toxicol. Environ. Chem. 2016, 99, 315–330. [Google Scholar] [CrossRef]
- Sánchez-Rubio, M.; Alnakip, M.E.A.; Abouelnaga, M.; Taboada-Rodriguez, A.; Marin-Iniesta, F. Use of thermosonication for inactivation of E. coli O157:H7 in fruit juices and fruit juice/reconstituted skim milk beverages. Acta Hortic. 2018, 1194, 267–274. [Google Scholar] [CrossRef]
- Nunes, B.V.; da Silva, C.N.; Bastos, S.C.; de Souza, V.R. Microbiological Inactivation by Ultrasound in Liquid Products. Food Bioprocess Technol. 2022, 15, 2185–2209. [Google Scholar] [CrossRef]
- Ramírez-Melo, L.M.; del SocorroCruz-Cansino, N.; Delgado-Olivares, L.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Hernández-Traspeña, J.L.; Suárez-Jacobo, Á. Optimization of antioxidant activity properties of a thermosonicated beetroot (Beta vulgaris L.) juice and further in vitro bioaccessibility comparison with thermal treatments. LWT 2022, 154, 112780. [Google Scholar] [CrossRef]
- Alabdali, T.A.M.; Icyer, N.C.; Ozkaya, G.U.; Durak, M.Z. Effect of Stand-Alone and Combined Ultraviolet and Ultrasound Treatments on Physicochemical and Microbial Characteristics of Pomegranate Juice. Appl. Sci. 2020, 10, 5458. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Arias-Rico, J.; Ariza-Ortega, J.A.; Alanís-García, E.; Cruz-Cansino, N. Effect of ultrasound on microbiological load and antioxidant properties of blackberry juice. J. Food Process. Preserv. 2018, 42, e13489. [Google Scholar] [CrossRef]
- Tremarin, A.; Canbaz, E.A.; Brandão, T.R.S.; Silva, C.L.M. Modelling Alicyclobacillus acidoterrestris inactivation in apple juice using thermosonication treatments. LWT 2019, 102, 159–163. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, D.; Xi, P.; Cai, T.; Cao, X.; Liu, H.; Li, J. Effects of temperature-controlled ultrasound treatment on sensory properties, physical characteristics and antioxidant activity of cloudy apple juice. LWT 2021, 142, 111030. [Google Scholar] [CrossRef]
- Li, J.; Cheng, H.; Liao, X.; Liu, D.; Xiang, Q.; Wang, J.; Chen, S.; Ye, X.; Ding, T. Inactivation of Bacillus subtilis and quality assurance in Chinese bayberry (Myrica rubra) juice with ultrasound and mild heat. LWT 2019, 108, 113–119. [Google Scholar] [CrossRef]
- Tomadoni, B.; Cassani, L.; Moreira, M.D.R.; Ponce, A.; Agüero, M.V. Natural Antimicrobials Combined with Ultrasound Treatments to Enhance Quality Parameters and Safety of Unpasteurized Strawberry Juice. Int. J. Fruit Sci. 2019, 20, S178–S197. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Sayago-Ayerdi, S.G.; Sánchez-Burgos, J.A.; Ramírez-Mares, M.V.; García-Magaña, M.; de Lourdes Montalvo-González, E. Effect of thermosonication on polyphenol oxidase inactivation and quality parameters of soursop nectar. LWT—Food Sci. Technol. 2017, 75, 545–551. [Google Scholar] [CrossRef]
- Park, J.J.; Olawuyi, I.F.; Lee, W.Y. Influence of Thermo-sonication and Ascorbic Acid Treatment on Microbial Inactivation and Shelf-Life Extension of Soft Persimmon (Diospyros kaki T.) Juice. Food Bioprocess Technol. 2021, 14, 429–440. [Google Scholar] [CrossRef]
- Demir, H.; Kılınç, A. Effect of batch and continuous thermosonication on the microbial and physicochemical quality of pumpkin juice. J. Food Sci. Technol. 2019, 56, 5036–5045. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Karolina De Araújo Barroso, M.; De Godoy, E.; Filho, A.; Andre, F.; Fernandes, N.; Rodrigues, S. Ultrasound and Ozone Processing of Cashew Apple Juice: Effects of Single and Combined Processing on the Juice Quality and Microbial Stability. Processes 2021, 9, 2243. [Google Scholar] [CrossRef]
- Sagong, H.-G.; Lee, S.-Y.; Chang, P.-S.; Heu, S.; Ryu, S.; Choi, Y.-J.; Kang, D.-H. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011, 145, 287–292. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Roohi, R.; Mahmoudi, M.R.; Granato, D. Modeling inactivation of Listeria monocytogenes, Shigella sonnei, Byssochlamys fulva and Saccharomyces cerevisiae and ascorbic acid and β-carotene degradation kinetics in tangerine juice by pulsed-thermosonication. LWT 2019, 111, 612–621. [Google Scholar] [CrossRef]
- Lalou, S.; Ordoudi, S.A.; Mantzouridou, F.T. On the Effect of Microwave Heating on Quality Characteristics and Functional Properties of Persimmon Juice and Its Residue. Foods 2021, 10, 2650. [Google Scholar] [CrossRef]
- Vignali, G.; Gozzi, M.; Pelacci, M.; Stefanini, R. Non-conventional Stabilization for Fruit and Vegetable Juices: Overview, Technological Constraints, and Energy Cost Comparison. Food Bioprocess Technol. 2022, 15, 1729–1747. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Deering, A.J.; San Martin-Gonzalez, M.F.; Campanella, O.H. Microwave pasteurization of apple juice: Modeling the inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium at 80–90 °C. Food Microbiol. 2020, 87, 103382. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.G.; Sun, D.W.; Han, Z.; Cheng, J.H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Tang, J.; Hong, Y.K.; Inanoglu, S.; Liu, F. Microwave pasteurization for ready-to-eat meals. Curr. Opin. Food Sci. 2018, 23, 133–141. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Lei, D.; Huang, Y.; Cheng, S.; Zhu, Z.; Wu, Z.; Cravotto, G. Impact of ultrasound, microwaves and high-pressure processing on food components and their interactions. Trends Food Sci. Technol. 2021, 109, 1–15. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef]
- Bilgin, Ö.; Çarli, U.; Erdoğan, S.; Emrah Maviş, M.; Göksu Gürsu, G.; Yilmaz, M. Karadeniz’de (Sinop Yarımadası Civarı) Avlanan İzmarit Balığı, Spicara smaris (Linnaeus, 1758), Etinin LC-MS/MS Kullanarak Amino Asit İçeriğinin Tespiti ve Ağırlık-Boy İlişkisi. Türk Tarım ve Doğa Bilim. Derg. 2019, 6, 130–136. [Google Scholar] [CrossRef]
- Sezer, B.; Apaydin, H.; Bilge, G.; Boyaci, I.H. Detection of Pistacia vera adulteration by using laser induced breakdown spectroscopy. J. Sci. Food Agric. 2019, 99, 2236–2242. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Zeng, X.A.; Sehrish; Shabbir, M.A.; Aadil, R.M. Combined effect of microwave and ultrasonication treatments on the quality and stability of sugarcane juice during cold storage. Int. J. Food Sci. Technol. 2019, 54, 2563–2569. [Google Scholar] [CrossRef]
- Traore, M.B.; Sun, A.; Gan, Z.; Senou, H.; Togo, J.; Fofana, K.H. Antimicrobial capacity of ultrasound and ozone for enhancing bacterial safety on inoculated shredded green cabbage (Brassica oleracea var. capitata). Can. J. Microbiol. 2020, 66, 125–137. [Google Scholar] [CrossRef]
- Hosseinzadeh Samani, B.; Khoshtaghaza, M.H.; Minaee, S.; Abbasi, S. Modeling the Simultaneous Effects of Microwave and Ultrasound Treatments on Sour Cherry Juice Using Response Surface Methodology. J. Agric. Sci. Technol. 2015, 17, 837–846. [Google Scholar]
- Hashemi, S.M.B.; Gholamhosseinpour, A.; Niakousari, M. Application of microwave and ohmic heating for pasteurization of cantaloupe juice: Microbial inactivation and chemical properties. J. Sci. Food Agric. 2019, 99, 4276–4286. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Mumtaz, S.; Lim, J.S.; Jang, J.H.; Kim, D.; Sahu, B.D.; Bogaerts, A.; Choi, E.H. Evaluation of non-thermal effect of microwave radiation and its mode of action in bacterial cell inactivation. Sci. Rep. 2021, 11, 14003. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Song, L.; Zhang, X.; Liu, D.; Hu, Y.; Guo, M. Inactivation of Staphylococcus aureus using ultrasound in combination with thyme essential oil nanoemulsions and its synergistic mechanism. LWT 2021, 147, 111574. [Google Scholar] [CrossRef]
- Soleimanzadeh, B.; Amoozandeh, A.; Shoferpour, M.; Yolmeh, M. New approaches to modeling Staphylococcus aureus inactivation by ultrasound. Ann. Microbiol. 2018, 68, 313–319. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhao, X.; Wang, W.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Inactivation of Staphylococcus aureus and Escherichia coli in milk by different processing sequences of ultrasound and heat. J. Food Saf. 2019, 39, e12614. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A.A.; Al Sheikh, Y.M.; Alaboudi, A.R.; Olaimat, A.N.; Al-Holy, M.; Al-Rousan, W.M.; Holley, R.; Jo, A.A.A. Inactivation of Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes in Tahini by Microwave Heating. Foods 2021, 10, 2972. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Mittal, G.S.; Griffiths, M.W. Inactivation of Salmonella enteritidis in Liquid Whole Egg using Combination Treatments of Pulsed Electric Field, High Pressure and Ultrasound. Biosyst. Eng. 2006, 94, 403–413. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020, 60, 104763. [Google Scholar] [CrossRef]
- Ferrario, M.; Guerrero, S. Effect of a continuous flow-through pulsed light system combined with ultrasound on microbial survivability, color and sensory shelf life of apple juice. Innov. Food Sci. Emerg. Technol. 2016, 34, 214–224. [Google Scholar] [CrossRef]
- Picouet, P.A.; Landl, A.; Abadias, M.; Castellari, M.; Viñas, I. Minimal processing of a Granny Smith apple purée by microwave heating. Innov. Food Sci. Emerg. Technol. 2009, 10, 545–550. [Google Scholar] [CrossRef]
- Das, M.J.; Das, A.J.; Chakraborty, S.; Baishya, P.; Ramteke, A.; Deka, S.C. Effects of microwave combined with ultrasound treatment on the pasteurization and nutritional properties of bottle gourd (Lagenaria siceraria) juice. J. Food Process. Preserv. 2020, 44, e14904. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; Gut, J.A.W.; Martinez, A.; Rodrigo, D. Inactivation kinetics of Escherichia coli O157:H7 and Listeria monocytogenes in apple juice by microwave and conventional thermal processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kang, J.-H.; Song, K. Bin Improving the Microbial Safety of Fresh-Cut Endive with a Combined Treatment of Cinnamon Leaf Oil Emulsion Containing Cationic Surfactants and Ultrasound. J. Microbiol. Biotechnol. 2018, 28, 503–509. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.; Ayim, I.; Aheto, J.H.; Hong, C.; Zhou, C. Reduction of Listeria innocua in fresh-cut Chinese cabbage by a combined washing treatment of sweeping frequency ultrasound and sodium hypochlorite. LWT 2019, 101, 410–418. [Google Scholar] [CrossRef]
- Nadeem, M.; Ghaffar, A.; Hashim, M.M.; Murtaza, M.A.; Ranjha, M.M.A.N.; Mehmood, A.; Riaz, M.N. Sonication and Microwave Processing of Phalsa Drink: A Synergistic Approach. Int. J. Fruit Sci. 2021, 21, 993–1007. [Google Scholar] [CrossRef]
- Campos, L.; Seixas, L.; Dias, S.; Peres, A.M.; Veloso, A.C.A.; Henriques, M. Effect of Extraction Method on the Bioactive Composition, Antimicrobial Activity and Phytotoxicity of Pomegranate By-Products. Foods 2022, 11, 992. [Google Scholar] [CrossRef]
- Erdal, B.; Yıkmış, S.; Demirok, N.T.; Bozgeyik, E.; Levent, O. Effects of Non-Thermal Treatment on Gilaburu Vinegar (Viburnum opulus L.): Polyphenols, Amino Acid, Antimicrobial, and Anticancer Properties. Biology 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Céspedes-Acuña, C.; Silva, F.L.; Alarcón-Enos, J. Improvement of the polyphenol extraction from avocado peel by assisted ultrasound and microwaves. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Sharma, M.; Adler, B.B.; Harrison, M.D.; Beuchat, L.R. Thermal tolerance of acid-adapted and unadapted Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes in cantaloupe juice and watermelon juice. Lett. Appl. Microbiol. 2005, 41, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, S.S.; Kang, D.H. Effect of pH for inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes in orange juice by ohmic heating. LWT - Food Sci. Technol. 2015, 62, 83–88. [Google Scholar] [CrossRef]
- Song, W.J.; Kang, D.H. Influence of water activity on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes in peanut butter by microwave heating. Food Microbiol. 2016, 60, 104–111. [Google Scholar] [CrossRef]
- Park, J.S.; Ha, J.W. Ultrasound treatment combined with fumaric acid for inactivating food-borne pathogens in apple juice and its mechanisms. Food Microbiol. 2019, 84, 103277. [Google Scholar] [CrossRef]
- Malinowska-Pańczyk, E.; Królik, K.; Skorupska, K.; Puta, M.; Martysiak-Żurowska, D.; Kiełbratowska, B. Microwave heat treatment application to pasteurization of human milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 42–48. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Peng, M.J.; Wang, Z.H.; Yang, Q.L.; Peng, S. Ultrasound-microwave assisted extraction of flavonoid compounds from Eucommia ulmoides leaves and an evaluation of their antioxidant and antibacterial activities. Arch. Biol. Sci. 2020, 72, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Optimizing the antioxidant biocompound recovery from peach waste extraction assisted by ultrasounds or microwaves. Sonochemistry 2019, 63, 104954. [Google Scholar] [CrossRef]
- Alonso-Carrillo, N.; Aguilar-Santamaría, M.; de los ÁngelesAguilar-Santamaría, M.; Vernon-Carter, E.J.; Jiménez-Alvarado, R.; Cruz-Sosa, F.; Román-Guerrero, A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crops Prod. 2017, 103, 213–221. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Study of continuous flow ultrasonication to improve total phenolic content and antioxidant activity in sorghum flour and its comparison with batch ultrasonication. Ultrason. Sonochem. 2021, 71, 105402. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Noman, A.; Qixing, J.; Xu, Y.; Abed, S.M.; Obadi, M.; Ali, A.H.; AL-Bukhaiti, W.Q.; Xia, W. Effects of ultrasonic, microwave, and combined ultrasonic-microwave pretreatments on the enzymatic hydrolysis process and protein hydrolysate properties obtained from Chinese sturgeon (Acipenser sinensis). J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Li, Z.; Jiang, Y.; Zhuang, W.; Zheng, B.; Tian, Y. Effects of ultrasonic pretreatments on thermodynamic properties, water state, color kinetics, and free amino acid composition in microwave vacuum dried lotus seeds. Dry. Technol. 2020, 38, 534–544. [Google Scholar] [CrossRef]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khaled Khodja, N.; Remini, H.; Madani, K.; Meléndez-Martínez, A.J. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Xie, B.; Sun, Z. Effect of ultrasound combined with ultraviolet treatment on microbial inactivation and quality properties of mango juice. Ultrason. Sonochem. 2020, 64, 105000. [Google Scholar] [CrossRef]
- Sánchez, E.; Sanjuán, N.; Cárcel, J.A.; Benedito, J. Aplicación de los ultrasonidos en las industrias de productos lácteos y derivados (y II): Ultrasonidos de alta intensidad. Aliment. Equipos Tecnol. 1998, 17, 135–141. [Google Scholar]
- Carvalho, L.R.F.; Souza, S.R.; Martinis, B.S.; Korn, M. Monitoring of the ultrasonic irradiation effect on the extraction of airborne particulate matter by ion chromatography. Anal. Chim. Acta 1995, 317, 171–179. [Google Scholar] [CrossRef]
- Pérez-Grijalba, B.; García-Zebadúa, J.C.; Ruíz-Pérez, V.M.; Téllez-Medina, D.I.; García-Pinilla, S.; Guzmán-Gerónimo, R.I.; Mora-Escobedo, R. Biofunctionality, colorimetric coefficients and microbiological stability of blackberry (Rubus fructicosus var. Himalaya) juice under microwave/ultrasound processing. Rev. Mex. Ing. Química 2018, 17, 13–28. [Google Scholar] [CrossRef]
- Yıkmış, S.; Aksu, H.; Çöl, B.G.; Alpaslan, M. Thermosonication processing of quince (Cydonia Oblonga) juice: Effects on total phenolics, ascorbic acid, antioxidant capacity, color and sensory properties. Ciência e Agrotecnologia 2019, 43, 1–15. [Google Scholar] [CrossRef]
- Lafarga, T.; Ruiz-Aguirre, I.; Abadias, M.; Viñas, I.; Bobo, G.; Aguiló-Aguayo, I. Effect of Thermosonication on the Bioaccessibility of Antioxidant Compounds and the Microbiological, Physicochemical, and Nutritional Quality of an Anthocyanin-Enriched Tomato Juice. Food Bioprocess Technol. 2019, 12, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour degradation and quality parameters of sonicated orange juice using response surface methodology. LWT - Food Sci. Technol. 2008, 41, 1876–1883. [Google Scholar] [CrossRef]
- Raju, S.; Deka, S.C. Influence of thermosonication treatments on bioactive compounds and sensory quality of fruit (Haematocarpus validus) juice. J. Food Process. Preserv. 2018, 42, e13701. [Google Scholar] [CrossRef]






| Independent Variable | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| U-time (min, X1 ) | 12 | 16 | 20 |
| Amplitude (%, X2 ) | 60 | 80 | 100 |
| M-time (s, X3) | 30 | 35 | 40 |
| Microwave power (Watt, X4) | 200 | 450 | 700 |
| Sample | Encoded Independent Variables | Dependent Variables (Log Reduction) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasound | Microwave | Response 1 | Response 2 | Response 3 | Response 4 | |||||||
| X1- Time (min) | X2- Amplitude (%) | X3- Time (s) | X4- Power (W) | E.coli ATCC 25922 (Log CFU/mL) | S. aureus ATCC 6538 (Log CFU/mL) | S. Enteritidis ATCC 13076 (Log CFU/mL) | L. monocytogenes DSM 12464 (Log CFU/mL) | |||||
| Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | |||||
| 1 | 12 | 60 | 30 | 700 | 3.81 | 3.61 | 3.75 | 3.93 | 3.88 | 4.00 | 5.88 | 6.03 |
| 2 | 20 | 100 | 40 | 700 | 6.68 | 6.32 | 6.36 | 6.33 | 6.07 | 6.10 | 8.00 | 8.16 |
| 3 | 20 | 100 | 30 | 200 | 0.44 | 0.42 | 0.48 | 0.49 | 0.55 | 0.53 | 1.55 | 1.55 |
| 4 | 12 | 100 | 40 | 700 | 6.25 | 6.13 | 6.13 | 6.24 | 5.86 | 5.99 | 8.00 | 8.15 |
| 5 | 20 | 80 | 35 | 450 | 1.85 | 1.86 | 1.72 | 1.96 | 2.00 | 2.23 | 3.78 | 3.87 |
| 6 | 20 | 60 | 40 | 700 | 6.23 | 6.13 | 6.11 | 6.26 | 5.84 | 5.99 | 8.00 | 8.14 |
| 7 | 20 | 100 | 30 | 700 | 3.93 | 3.85 | 3.85 | 4.16 | 3.97 | 4.15 | 6.03 | 6.12 |
| 8 | 16 | 80 | 35 | 450 | 1.83 | 1.73 | 1.70 | 1.67 | 1.98 | 1.94 | 3.85 | 3.90 |
| 9 | 12 | 60 | 40 | 200 | 0.58 | 0.50 | 0.51 | 0.39 | 0.63 | 0.60 | 1.59 | 1.64 |
| 10 | 20 | 60 | 30 | 200 | 0.35 | 0.31 | 0.22 | 0.29 | 0.43 | 0.44 | 1.5 | 1.48 |
| 11 | 16 | 80 | 35 | 450 | 1.83 | 1.73 | 1.70 | 1.67 | 1.98 | 1.94 | 3.85 | 3.90 |
| 12 | 16 | 80 | 35 | 450 | 1.83 | 1.73 | 1.70 | 1.67 | 1.98 | 1.94 | 3.85 | 3.90 |
| 13 | 16 | 80 | 35 | 450 | 1.83 | 1.73 | 1.70 | 1.67 | 1.98 | 1.94 | 3.85 | 3.90 |
| 14 | 16 | 80 | 35 | 450 | 1.83 | 1.73 | 1.70 | 1.67 | 1.98 | 1.94 | 3.85 | 3.90 |
| 15 | 12 | 80 | 35 | 450 | 1.83 | 1.77 | 1.72 | 1.88 | 2.03 | 2.18 | 3.84 | 3.85 |
| 16 | 16 | 80 | 35 | 200 | 0.56 | 0.55 | 0.49 | 0.55 | 0.61 | 0.64 | 2.01 | 2.07 |
| 17 | 20 | 60 | 40 | 200 | 0.60 | 0.54 | 0.54 | 0.45 | 0.64 | 0.63 | 1.64 | 1.64 |
| 18 | 20 | 100 | 40 | 200 | 0.63 | 0.67 | 0.56 | 0.56 | 0.67 | 0.70 | 1.65 | 1.66 |
| 19 | 16 | 60 | 35 | 450 | 1.83 | 1.76 | 1.73 | 1.86 | 1.96 | 2.07 | 3.84 | 3.89 |
| 20 | 16 | 100 | 35 | 450 | 1.86 | 1.88 | 1.74 | 1.99 | 1.87 | 2.15 | 3.89 | 3.93 |
| 21 | 12 | 100 | 30 | 700 | 3.87 | 3.71 | 3.82 | 4.07 | 3.94 | 4.08 | 5.95 | 6.09 |
| 22 | 16 | 80 | 30 | 450 | 0.97 | 0.79 | 0.81 | 0.44 | 0.81 | 0.9 | 2.73 | 2.88 |
| 23 | 20 | 60 | 30 | 700 | 3.85 | 3.68 | 3.78 | 4.00 | 3.90 | 4.03 | 5.90 | 6.05 |
| 24 | 16 | 80 | 35 | 450 | 1.83 | 1.73 | 1.70 | 1.67 | 1.98 | 1.94 | 3.85 | 3.90 |
| 25 | 12 | 100 | 30 | 200 | 0.42 | 0.36 | 0.38 | 0.41 | 0.49 | 0.49 | 1.51 | 1.51 |
| 26 | 16 | 80 | 35 | 700 | 5.11 | 5.02 | 4.90 | 5.28 | 4.73 | 5.12 | 7.53 | 7.61 |
| 27 | 12 | 60 | 30 | 200 | 0.18 | 0.32 | 0.05 | 0.23 | 0.36 | 0.45 | 1.47 | 1.45 |
| 28 | 16 | 80 | 35 | 450 | 1.83 | 1.73 | 1.70 | 1.67 | 1.98 | 1.94 | 3.85 | 3.90 |
| 29 | 16 | 80 | 40 | 450 | 1.98 | 2.11 | 0.83 | 1.6 | 1.66 | 1.95 | 4.04 | 3.99 |
| 30 | 12 | 60 | 40 | 700 | 6.21 | 6.01 | 6.03 | 6.18 | 5.79 | 5.93 | 8.00 | 8.15 |
| 31 | 12 | 100 | 40 | 200 | 0.61 | 0.56 | 0.55 | 0.48 | 0.64 | 0.63 | 1.64 | 1.64 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value | Adj SS | Adj MS | F-Value | p-Value | Adj SS | Adj MS | F-Value | p-Value | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E.coli ATCC 25922 | S. aureus ATCC 6538 | S. Enteritidis ATCC 13076 | L. monocytogenes DSM 12464 | ||||||||||||||
| Model | 14 | 116.80 | 8.34 | 754.12 | 0.000 | 114.461 | 8.1758 | 127.77 | 0.000 | 98.99 | 7.07 | 387.14 | 0.000 | 144.41 | 10.32 | 4750.77 | 0.000 |
| Linear | 4 | 103.91 | 25.98 | 2348.09 | 0.000 | 99.394 | 24.8484 | 388.34 | 0.000 | 89.49 | 22.37 | 1224.89 | 0.000 | 137.57 | 34.39 | 15839.34 | 0.000 |
| 1 | 0.04 | 0.04 | 3.2 | 0.092 | 0.025 | 0.0252 | 0.39 | 0.539 | 0.01 | 0.01 | 0.6 | 0.450 | 0.00 | 0.00 | 0.74 | 0.402 | |
| 1 | 0.06 | 0.06 | 5.48 | 0.033 | 0.071 | 0.0713 | 1.11 | 0.307 | 0.02 | 0.02 | 1.25 | 0.279 | 0.01 | 0.01 | 4.38 | 0.053 | |
| 1 | 7.90 | 7.90 | 714.24 | 0.000 | 6.127 | 6.1266 | 95.75 | 0.000 | 4.97 | 4.97 | 272.32 | 0.000 | 5.60 | 5.60 | 2577.92 | 0.000 | |
| 1 | 95.91 | 95.91 | 8669.42 | 0.000 | 93.17 | 93.1705 | 1456.09 | 0.000 | 84.48 | 84.48 | 4625.37 | 0.000 | 131.96 | 131.96 | 60774.32 | 0.000 | |
| Square | 4 | 7.96 | 1.99 | 179.78 | 0.000 | 10.648 | 2.662 | 41.60 | 0.000 | 6.33 | 1.58 | 86.63 | 0.000 | 3.09 | 0.77 | 355.81 | 0.000 |
| 1 | 0.02 | 0.02 | 1.69 | 0.212 | 0.163 | 0.1627 | 2.54 | 0.130 | 0.18 | 0.18 | 10.08 | 0.006 | 0.01 | 0.01 | 2.11 | 0.165 | |
| 1 | 0.02 | 0.02 | 2.1 | 0.167 | 0.175 | 0.1754 | 2.74 | 0.117 | 0.07 | 0.07 | 3.99 | 0.063 | 0.00 | 0.00 | 0.14 | 0.714 | |
| 1 | 0.20 | 0.20 | 17.75 | 0.001 | 1.095 | 1.0953 | 17.12 | 0.001 | 0.69 | 0.69 | 37.6 | 0.000 | 0.57 | 0.57 | 261.29 | 0.000 | |
| 1 | 3.05 | 3.05 | 275.66 | 0.000 | 3.895 | 3.8951 | 60.87 | 0.000 | 2.20 | 2.20 | 120.22 | 0.000 | 2.18 | 2.18 | 1004.32 | 0.000 | |
| 2-Way Interaction | 6 | 4.94 | 0.82 | 74.36 | 0.000 | 4.419 | 0.7366 | 11.51 | 0.000 | 3.18 | 0.53 | 28.98 | 0.000 | 3.76 | 0.63 | 288.36 | 0.000 |
| 1 | 0.01 | 0.00 | 0.43 | 0.519 | 0 | 0.0002 | 0.00 | 0.951 | 0.00 | 0.00 | 0.11 | 0.742 | 0.00 | 0.00 | 0.12 | 0.737 | |
| 1 | 0.00 | 0.00 | 0.24 | 0.631 | 0 | 0 | 0.00 | 0.987 | 0.00 | 0.00 | 0.05 | 0.822 | 0.00 | 0.00 | 0.37 | 0.552 | |
| 1 | 0.01 | 0.01 | 0.5 | 0.492 | 0 | 0.0003 | 0.00 | 0.949 | 0.00 | 0.00 | 0.08 | 0.777 | 0.00 | 0.00 | 0.03 | 0.860 | |
| 1 | 0.00 | 0.00 | 0.03 | 0.874 | 0.007 | 0.0066 | 0.10 | 0.752 | 0.00 | 0.00 | 0.01 | 0.920 | 0.00 | 0.00 | 1.5 | 0.239 | |
| 1 | 0.00 | 0.00 | 0.34 | 0.566 | 0.002 | 0.0018 | 0.03 | 0.868 | 0.00 | 0.00 | 0.06 | 0.811 | 0.00 | 0.00 | 0.03 | 0.866 | |
| 1 | 4.92 | 4.92 | 444.6 | 0.000 | 4.411 | 4.4105 | 68.93 | 0.000 | 3.17 | 3.17 | 173.54 | 0.000 | 3.75 | 3.75 | 1728.13 | 0.000 | |
| Error | 16 | 0.18 | 0.01 | 1.024 | 0.064 | 0.29 | 0.02 | 0.04 | 0.00 | ||||||||
| Lack of Fit | 10 | 0.18 | 0.02 | * | * | 1.024 | 0.1024 | * | * | 0.29 | 0.03 | * | * | 0.04 | 0.00 | * | * |
| Pure Error | 6 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | ||||||||
| Total | 30 | ||||||||||||||||
| 99.85% | 99.11% | 99.71% | 99.98% | ||||||||||||||
| Adj | 99.72% | 98.34% | 99.45% | 99.95% | |||||||||||||
| Pred. | 99.07% | 96.28% | 98.95% | 99.89% | |||||||||||||
| Variable | Setting | |||
|---|---|---|---|---|
| X1- Time (min) | 12 | |||
| X2- Amplitude (%) | 60 | |||
| X3- Time (s) | 34 | |||
| X4- Power (W) | 700 | |||
| Response (UM-TJ) | Fit | SE Fit | 95% CI | 95% PI |
| E.coli ATCC 25922 (log CFU/mL) | 5.00 | 0.0909 | (4.8072; 5.1928) | (4.7052; 5.2947) |
| S. aureus ATCC 6538 (log CFU/mL) | 5.27 | 0.219 | (4.803; 5.730) | (4.558; 5.975) |
| S. Enteritidis ATCC 13076 (log CFU/mL) | 5.12 | 0.117 | (4.868; 5.363) | (4.737; 5.494) |
| L. monocytogenes DSM 12464 (log CFU/mL) | 7.19 | 0.0403 | (7.1021; 7.2729) | (7.0569; 7.3181) |
| Amino Acid Content (mg/100 mL) | Samples | ||
|---|---|---|---|
| C-TJ | P-TJ | UM-TJ | |
| Alanine (Ala) | 13.84 ± 0.01 b | 10.68 ± 0.02 a | 18.79 ± 0.01 c |
| Arginine (Arg) | 105.58 ± 0.1 b | 90.59 ± 0.16 a | 115.64 ± 0.36 c |
| Aspartic Acid (Asp) | 46.22 ± 0.07 b | 25.97 ± 0.01 a | 80.69 ± 0.04 a |
| Cysteine (Cys) | n.d | n.d | 0.01 ± 0.01 |
| Glutamic Acid (Glu) | 7.74 ± 0.01 b | 4.78 ± 0.00 a | 13.45 ± 0.01 a |
| Glycine (Gly) | 1.39 ± 0.01 a | 1.46 ± 0.00 b | 1.53 ± 0.00 c |
| Histidine (His) | n.d | n.d | 0.22 ± 0.00 |
| Isoleucine (Ile) | 0.17 ± 0.01 b | 0.08 ± 0.00 a | 1.00 ± 0.00 c |
| Leucine (Leu) | 0.55 ± 0.01 b | 0.43 ± 0.00 a | 2.01 ± 0.00 c |
| Lysine (Lys) | 1.55 ± 0.01 b | 0.75 ± 0.00 a | 7.07 ± 0.01 c |
| Methionine (Met) | 0.15 ± 0.01 b | 0.10 ± 0.00 a | 0.69 ± 0.01 c |
| Ornithine (Orn) | 3.27 ± 0.00 b | 2.65 ± 0.01 a | 3.86 ± 0.01 c |
| Phenylalanine (Phe) | 0.43 ± 0.01 a | 0.33 ± 0.01 a | 2.43 ± 0.05 b |
| Proline (Pro) | 202.41 ± 0.06 b | 143.09 ± 0.09 a | 220.40 ± 0.24 c |
| Serine (Ser) | 13.26 ± 0.00 b | 9.60 ± 0.00 a | 16.77 ± 0.06 c |
| Threonine (Thr) | 2.57 ± 0.01 b | 1.51 ± 0.02 a | 3.85 ± 0.01 c |
| Tyrosine (Tyr) | 0.82 ± 0.00 b | 0.31 ± 0.00 a | 1.68 ± 0.00 c |
| Valine (Val) | 1.12 ± 0.00 b | 0.70 ± 0.00 a | 3.01 ± 0.00 c |
| Totals (mg/100 mL) | 401.02 ± 0.04 a | 293.01 ± 0.23 b | 493.05 ± 0.48 c |
| Minerals (Minerals (mg/L) | Samples | ||
|---|---|---|---|
| C-TJ | P-TJ | UM-TJ | |
| Ag | n.d | n.d | n.d |
| Al | 0.05 ± 0.00 a | n.d | 0.05 ± 0.00 a |
| Ca | 125.49 ± 0.01 a | 106.45 ± 0.01 b | 81.16 ± 0.01 c |
| Cd | n.d | n.d | n.d |
| Co | n.d | n.d | n.d |
| Cr | n.d | n.d | n.d |
| Cu | 0.60 ± 0.01 b | 0.26 ± 0.00 c | 1.05 ± 0.00 a |
| Fe | 1.68 ± 0.01 a | 0.78 ± 0.15 b | 1.13 ± 0.01 b |
| K | 1834.16 ± 0.06 c | 2281.62 ± 0.10 a | 1981.56 ± 0.16 b |
| Mg | 41.96 ± 0.03 b | 27.96 ± 0.02 c | 54.16 ± 0.01 a |
| Mn | 0.27 ± 0.00 a | 0.17 ± 0.00 b | 0.26 ± 0.00 a |
| Na | 9.32 ± 0.00 b | 8.12 ± 0.01 c | 9.46 ± 0.00 a |
| Ni | n.d | n.d | n.d |
| Pb | n.d | n.d | n.d |
| Zn | 0.64 ± 0.00 b | 0.46 ± 0.00 c | 0.86 ± 0.01 a |
| Analyzes | Samples | |||
|---|---|---|---|---|
| C-TJ | P-TJ | UM-TJ | ||
| Color | L* | 50.24 ± 0.74 a | 52.85 ± 0.72 b | 55.72 ± 0.61 c |
| a* | 12.36 ± 0.58 b | 9.90 ± 0.42 a | 11.30 ± 0.36 b | |
| b* | 14.46 ± 0.25 b | 11.26 ± 0.28 a | 15.50 ± 0.12 c | |
| Chroma (C) | 19.02 ± 0.56 b | 15.00 ± 0.47 a | 19.19 ± 0.21 b | |
| Hue angle (h°) | 49.49 ± 0.85 a | 48.68 ± 0.69 a | 53.91 ± 0.94 b | |
| ΔE | - | 4.88 ± 0.42 | 5.59 ± 1.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirok, N.T.; Yıkmış, S. Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens. Processes 2022, 10, 2100. https://doi.org/10.3390/pr10102100
Demirok NT, Yıkmış S. Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens. Processes. 2022; 10(10):2100. https://doi.org/10.3390/pr10102100
Chicago/Turabian StyleDemirok, Nazan Tokatlı, and Seydi Yıkmış. 2022. "Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens" Processes 10, no. 10: 2100. https://doi.org/10.3390/pr10102100