Effect of Chain Structure on the Various Properties of the Copolymers of Fluorinated Norbornenes with Cyclooctene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monomer Synthesis
2.3. Homopolymer Synthesis
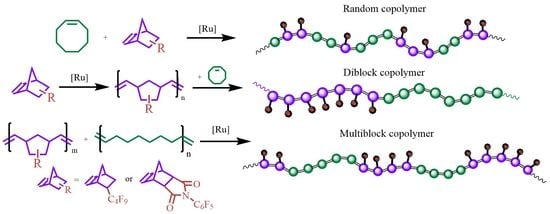
2.4. Synthesis of NBFD-COE and NBF–COE Copolymers with Different Chain Structure
2.5. Characterization
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
3.2. Thermal and Surface Properties
3.3. Gas Transport Properties
3.4. Light Scattering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okamoto, Y.; Chiang, H.-C.; Fang, M.; Galizia, M.; Merkel, T.; Yavari, M.; Nguyen, H.; Lin, H. Perfluorodioxolane Polymers for Gas Separation Membrane Applications. Membranes 2020, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Chiang, H.-C.; Merkel, T. Perfluoropolymers for Gas Separation Membrane Applications. In Fascinating Fluoropolymers and Their Applications; Ameduri, B., Fomin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–155. ISBN 978-0-12-821873-0. [Google Scholar]
- Cetina-Mancilla, E.; González-Díaz, M.O.; Sulub-Sulub, R.; Zolotukhin, M.G.; González-Díaz, A.; Herrera-Kao, W.; Ruiz-Treviño, F.A.; Aguilar-Vega, M. Aging Resistant, Fluorinated Aromatic Polymers with Ladderized, Rigid Kink-Structured Backbones for Gas Separations. J. Memb. Sci. 2022, 659, 120764. [Google Scholar] [CrossRef]
- Yampolskii, Y.P.; Belov, N.A.; Alentiev, A.Y. Fluorine in the Structure of Polymers: Influence on the Gas Separation Properties. Russ. Chem. Rev. 2019, 88, 387–405. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N.; Alentiev, A. Perfluorinated Polymers as Materials of Membranes for Gas and Vapor Separation. J. Memb. Sci. 2020, 598, 117779. [Google Scholar] [CrossRef]
- Belov, N.; Yampolskii, Y. Chapter 6—Gas Transport in Fluorine-Containing Polymers. In Fascinating Fluoropolymers and Their Applications; Ameduri, B., Fomin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–203. ISBN 978-0-12-821873-0. [Google Scholar]
- Wu, W.; Niu, H.; Lai, S.; Liu, C.; Zhou, L.; Huang, X. Synthesis, Characterization, and Gas Separation Properties of Novel Fluorinated Co-Polyimides with Bulky Side Groups. Polymer 2022, 257, 125273. [Google Scholar] [CrossRef]
- Améduri, B. The Promising Future of Fluoropolymers. Macromol. Chem. Phys. 2020, 221, 1900573. [Google Scholar] [CrossRef]
- Meier, S.; Stelzer, F.; Reisinger, H.; Haag, R.; Mecking, S.; Mülhaupt, R. Carbohydrate Analogue Polymers by Ring Opening Metathesis Polymerisation (ROMP) and Subsequent Catalytic Dihydroxylation. Chem. Commun. 2001, 9, 855–856. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Lodge, T.P. Synthesis and Self-Assembly of Fluorinated Block Copolymers. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1–8. [Google Scholar] [CrossRef]
- Dolui, S.; Kumar, D.; Banerjee, S.; Ameduri, B. Well-Defined Fluorinated Copolymers: Current Status and Future Perspectives. Accounts Mater. Res. 2021, 2, 242–251. [Google Scholar] [CrossRef]
- Nunes, S.P. Chapter 11—Block Copolymer Membranes. In Sustainable Nanoscale Engineering; Szekely, G., Livingston, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–316. ISBN 978-0-12-814681-1. [Google Scholar]
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 2017, 50, 3–22. [Google Scholar] [CrossRef]
- Beyer, V.P.; Kim, J.; Becer, C.R. Synthetic Approaches for Multiblock Copolymers. Polym. Chem. 2020, 11, 1271–1291. [Google Scholar] [CrossRef]
- Engelis, N.G.; Anastasaki, A.; Nurumbetov, G.; Truong, N.P.; Nikolaou, V.; Shegiwal, A.; Whittaker, M.R.; Davis, T.P.; Haddleton, D.M. Sequence-Controlled Methacrylic Multiblock Copolymers via Sulfur-Free RAFT Emulsion Polymerization. Nat. Chem. 2017, 9, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Gody, G.; Perrier, S. Preparation of Complex Multiblock Copolymers via Aqueous RAFT Polymerization at Room Temperature. Polym. Chem. 2015, 6, 4875–4886. [Google Scholar] [CrossRef]
- Lutz, J.-F. Writing on Polymer Chains. Acc. Chem. Res. 2013, 46, 2696–2705. [Google Scholar] [CrossRef]
- Soeriyadi, A.H.; Boyer, C.; Nyström, F.; Zetterlund, P.B.; Whittaker, M.R. High-Order Multiblock Copolymers via Iterative Cu(0)-Mediated Radical Polymerizations (SET-LRP): Toward Biological Precision. J. Am. Chem. Soc. 2011, 133, 11128–11131. [Google Scholar] [CrossRef]
- Alsubaie, F.; Anastasaki, A.; Wilson, P.; Haddleton, D.M. Sequence-Controlled Multi-Block Copolymerization of Acrylamides via Aqueous SET-LRP at 0 °C. Polym. Chem. 2015, 6, 406–417. [Google Scholar] [CrossRef]
- Ueda, M. Sequence Control in One-Step Condensation Polymerization. Prog. Polym. Sci. 1999, 24, 699–730. [Google Scholar] [CrossRef]
- Chum, P.S.; Swogger, K.W. Olefin Polymer Technologies—History and Recent Progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Litmanovich, A.D.; Platé, N.A.; Kudryavtsev, Y.V. Reactions in Polymer Blends: Interchain Effects and Theoretical Problems. Prog. Polym. Sci. 2002, 27, 915–970. [Google Scholar] [CrossRef]
- Maeda, T.; Otsuka, H.; Takahara, A. Dynamic Covalent Polymers: Reorganizable Polymers with Dynamic Covalent Bonds. Prog. Polym. Sci. 2009, 34, 581–604. [Google Scholar] [CrossRef]
- Ayres, N.; Weck, M. Supramolecular and Dynamic Covalent Polymers. Polym. Chem. 2012, 3, 3031–3032. [Google Scholar] [CrossRef]
- Roy, A.; Yu, X.; Dunn, S.; McGrath, J.E. Influence of Microstructure and Chemical Composition on Proton Exchange Membrane Properties of Sulfonated–Fluorinated, Hydrophilic–Hydrophobic Multiblock Copolymers. J. Memb. Sci. 2009, 327, 118–124. [Google Scholar] [CrossRef]
- Lee, H.-S.; Roy, A.; Lane, O.; Lee, M.; McGrath, J.E. Synthesis and Characterization of Multiblock Copolymers Based on Hydrophilic Disulfonated Poly(Arylene Ether Sulfone) and Hydrophobic Partially Fluorinated Poly(Arylene Ether Ketone) for Fuel Cell Applications. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 214–222. [Google Scholar] [CrossRef]
- Ahn, S.M.; Kim, T.H.; Yuk, J.; Jeong, H.Y.; Yu, D.M.; Hong, S.-K.; Hong, Y.T.; Lee, J.-C.; Kim, T.-H. Perfluorocyclobutyl-Containing Multiblock Copolymers to Induce Enhanced Hydrophilic/Hydrophobic Phase Separation and High Proton Conductivity at Low Humidity. J. Memb. Sci. 2022, 641, 119892. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, R.; Lee, C.H.; Lee, M.; McGrath, J.E. Partly Fluorinated Poly(Arylene Ether Ketone Sulfone) Hydrophilic–Hydrophobic Multiblock Copolymers for Fuel Cell Membranes. Int. J. Hydrogen Energy 2012, 37, 6132–6139. [Google Scholar] [CrossRef]
- Assumma, L.; Iojoiu, C.; Mercier, R.; Lyonnard, S.; Nguyen, H.D.; Planes, E. Synthesis of Partially Fluorinated Poly(Arylene Ether Sulfone) Multiblock Copolymers Bearing Perfluorosulfonic Functions. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1941–1956. [Google Scholar] [CrossRef]
- Pospiech, D.; Häußler, L.; Eckstein, K.; Komber, H.; Voigt, D.; Jehnichen, D.; Friedel, P.; Gottwald, A.; Kollig, W.; Kricheldorf, H.R. Synthesis and Phase Separation Behaviour of High Performance Multiblock Copolymers. High Perform. Polym. 2001, 13, S275–S292. [Google Scholar] [CrossRef]
- Gringolts, M.L.; Denisova, Y.I.; Shandryuk, G.A.; Krentsel, L.B.; Litmanovich, A.D.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Synthesis of Norbornene-Cyclooctene Copolymers by the Cross-Metathesis of Polynorbornene with Polyoctenamer. RSC Adv. 2015, 5, 316–319. [Google Scholar] [CrossRef]
- Morontsev, A.A.; Gringolts, M.L.; Filatova, M.P.; Peregudov, A.S.; Akmalov, T.R.; Masoud, S.M.; Osipov, S.N.; Denisova, Y.I.; Kudryavtsev, Y.V. Ruthenium–Carbene Complexes in the Synthesis of Polybutadiene and Its Cross-Metathesis with Polynorbornene. Polym. Sci. Ser. C 2019, 61, 65–75. [Google Scholar] [CrossRef]
- Gringolts, M.L.; Denisova, Y.I.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Olefin Metathesis in Multiblock Copolymer Synthesis. Beilstein J. Org. Chem. 2019, 15, 218–235. [Google Scholar] [CrossRef]
- Denisova, Y.I.; Roenko, A.V.; Adzhieva, O.A.; Gringolts, M.L.; Shandryuk, G.A.; Peregudov, A.S.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Facile Synthesis of Norbornene-Ethylene-Vinyl Acetate/Vinyl Alcohol Multiblock Copolymers by the Olefin Cross-Metathesis of Polynorbornene with Poly(5-Acetoxy-1-Octenylene). Polym. Chem. 2020, 11, 7063–7077. [Google Scholar] [CrossRef]
- Roenko, A.V.; Nikiforov, R.Y.; Gringolts, M.L.; Belov, N.A.; Denisova, Y.I.; Shandryuk, G.A.; Bondarenko, G.N.; Kudryavtsev, Y.V.; Finkelshtein, E.S. Olefin-Metathesis-Derived Norbornene-Ethylene-Vinyl Acetate/Vinyl Alcohol Multiblock Copolymers: Impact of the Copolymer Structure on the Gas Permeation Properties. Polymers 2022, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, E. Recent Developments in the Synthesis of Fluorinated Homopolymers and Block Copolymers via Living Ring Opening Metathesis Polymerisation. In Metathesis Polymerization of Olefins and Polymerization of Alkynes; Imamoglu, Y., Ed.; Springer: Cham, Switzerland, 1998; pp. 265–276. ISBN 978-94-011-5188-7. [Google Scholar]
- James Feast, W.; Khosravi, E. Synthesis of Fluorinated Polymers via ROMP: A Review. J. Fluor. Chem. 1999, 100, 117–125. [Google Scholar] [CrossRef]
- Vargas, J.; Santiago, A.A.; Gaviño, R.; Cerda, A.M.; Tlenkopatchev, M.A. Synthesis and Ring-Opening Metathesis Polymerization (ROMP) of New N -Fluoro-Phenylnorbornene Dicarboximides by 2nd Generation Ruthenium Alkylidene Catalysts. Express Polym. Lett. 2007, 1, 274–282. [Google Scholar] [CrossRef]
- Santiago, A.A.; Vargas, J.; Cruz-Gómez, J.; Tlenkopatchev, M.A.; Gaviño, R.; López-González, M.; Riande, E. Synthesis and Ionic Transport of Sulfonated Ring-Opened Polynorbornene Based Copolymers. Polymer 2011, 52, 4208–4220. [Google Scholar] [CrossRef]
- Santiago, A.A.; Vargas, J.; Cruz-Morales, J.A.; Tlenkopatchev, M.A.; Gaviño, R.; Malkanduev, Y.A.; Sivov, N.A. Synthesis of New Polymer Ionomers via Ring-Opening Metathesis Polymerization. Open J. Org. Polym. Mater. 2014, 04, 84–91. [Google Scholar] [CrossRef]
- Gnanasekaran, D.; Madrhavan, K.; Tsibouklis, J.; Reddy, B. Nanocomposites Based on Copolymers of Fluorinated Imide and Polyhedral Oligomeric Silsesquioxane Macromonomer: Microstructure and Morphology Studies. Polym. Int. 2013, 62, 190–195. [Google Scholar] [CrossRef]
- Schaefer, M.; Hanik, N.; Kilbinger, A.F.M. ROMP Copolymers for Orthogonal Click Functionalizations. Macromolecules 2012, 45, 6807–6818. [Google Scholar] [CrossRef]
- Wewerka, K.; Wewerka, A.; Stelzer, F.; Gallot, B.; Andruzzi, L.; Galli, G. New Microphase-Separated Diblock Copolymers Carrying Semifluorinated Side Groups Prepared by ROMP. Macromol. Rapid Commun. 2003, 24, 906–910. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Starannikova, L.E.; Sterlin, S.R.; Tyutyunov, A.A.; Tavtorkin, A.N.; Yampolskii, Y.P.; Finkelshtein, E.S. Synthesis and Gas-Separation Properties of Metathesis Poly(3-Fluoro-3-Pentafluoroethyl-4,4-Bis(Trifluoromethyl)Tricyclonene-7). Pet. Chem. 2015, 55, 753–758. [Google Scholar] [CrossRef]
- Borisov, I.L.; Akmalov, T.R.; Ivanov, A.O.; Volkov, V.V.; Finkelshtein, E.S.; Bermeshev, M. A New Cycloadduct Based on Quadricyclane and Perfluorocyclohexene: Synthesis, Metathesis Polymerization and Gas-Transport Properties of the Obtained Polymer. Mendeleev Commun. 2016, 26, 124–126. [Google Scholar] [CrossRef]
- Karpov, G.O.; Bermeshev, M.V.; Borisov, I.L.; Sterlin, S.R.; Tyutyunov, A.A.; Yevlampieva, N.P.; Bulgakov, B.A.; Volkov, V.V.; Finkelshtein, E.S. Metathesis-Type Poly-Exo-Tricyclononenes with Fluoroorganic Side Substituents: Synthesis and Gas-Transport Properties. Polymer 2018, 153, 626–636. [Google Scholar] [CrossRef]
- Karpov, G.O.; Alentiev, D.A.; Wozniak, A.I.; Bermesheva, E.V.; Lounev, I.V.; Gusev, Y.A.; Shantarovich, V.P.; Bermeshev, M.V. Dielectric Properties of Addition and Metathesis Polynorbornenes with Bulky Side-Substituents. Polymer 2020, 203, 122759. [Google Scholar] [CrossRef]
- Tashiro, K.; Akiyama, M.; Kashiwagi, K.; Okazoe, T. The Fluorocarbene Exploit: Enforcing Alternation in Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc. 2023, 145, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Pulamagatta, B.; Ostas, E.; Herbst, F.; Struth, B.; Binder, W.H. Shear Induced Structure Orientation in Norbornene Block Copolymers: In Situ Rheo-SAXS Investigations. Eur. Polym. J. 2012, 48, 1127–1134. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, Y.; Choi, T.-L.; Lim, J.; Char, K. Swelling-Induced Pore Generation in Fluorinated Polynorbornene Block Copolymer Films. Polym. Chem. 2018, 9, 3536–3542. [Google Scholar] [CrossRef]
- Han, H.; Zhou, D.; Ren, Q.; Ma, F.; Ma, C.; Xie, M. High-Performance All-Polymer Dielectric and Electrical Energy Storage Materials Containing Conjugated Segment and Multi-Fluorinated Pendants. Eur. Polym. J. 2020, 122, 109376. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lin, Y.-H.; Chiang, T.-C.; Yu, C.-Y. Synthesis of the Polymers Containing Norbornene and Tetraphenylethene by Ring-Opening Metathesis Polymerization and Their Structural Characterization, Aggregation-Induced Emission and Aniline Detection. Polymer 2022, 260, 125374. [Google Scholar] [CrossRef]
- Ahmed, E.; Womble, C.T.; Cho, J.; Dancel-Manning, K.; Rice, W.J.; Jang, S.S.; Weck, M. One-Pot Synthesis of Linear Triblock Terpolymers and Their Aqueous Self-Assembly. Polym. Chem. 2021, 12, 1967–1974. [Google Scholar] [CrossRef]
- Varlas, S.; Lawrenson, S.B.; Arkinstall, L.A.; O’Reilly, R.K.; Foster, J.C. Self-Assembled Nanostructures from Amphiphilic Block Copolymers Prepared via Ring-Opening Metathesis Polymerization (ROMP). Prog. Polym. Sci. 2020, 107, 101278. [Google Scholar] [CrossRef]
- Adzhieva, O.A.; Nikiforov, R.Y.; Gringolts, M.L.; Belov, N.A.; Filatova, M.P.; Denisova, Y.I.; Kudryavtsev, Y.V. Synthesis and Gas Separation Properties of Metathesis Poly(5-Perfluorobutyl-2-Norbornene). Polym. Sci. Ser. A 2022, 64, 424–433. [Google Scholar] [CrossRef]
- Santiago, A.A.; Vargas, J.; Fomine, S.; Gaviño, R.; Tlenkopatchev, M.A. Polynorbornene with Pentafluorophenyl Imide Side Chain Groups: Synthesis and Sulfonation. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Denisova, Y.I.; Zhigarev, V.A.; Gringolts, M.L.; Shandryuk, G.A.; Peregudov, A.S.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Cyclododecene in Olefin Metathesis: Polymerization and Macromolecular Cross-Metathesis with Polynorbornene. Polym. Sci. Ser. C 2019, 61, 120–133. [Google Scholar] [CrossRef]
- Pitet, L.M.; Zhang, J.; Hillmyer, M.A. Sequential ROMP of Cyclooctenes as a Route to Linear Polyethylene Block Copolymers. Dalt. Trans. 2013, 42, 9079–9088. [Google Scholar] [CrossRef] [PubMed]
- Denisova, Y.I.; Gringolts, M.L.; Peregudov, A.S.; Krentsel, L.B.; Litmanovich, E.A.; Litmanovich, A.D.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Cross-Metathesis of Polynorbornene with Polyoctenamer: A Kinetic Study. Beilstein J. Org. Chem. 2015, 11, 1796–1808. [Google Scholar] [CrossRef]
- Denisova, Y.I.; Gringolts, M.L.; Krentsel’, L.B.; Shandryuk, G.A.; Peregudov, A.S.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Synthesis of New Multiblock Copolymers via Cross-Metathesis Reaction of Polytrimethylsilylnorbornene and Polycyclooctene. Polym. Sci. Ser. B 2017, 59, 412–420. [Google Scholar] [CrossRef]
- Schneider, W.A.; Müller, M.F. Crystallinity and Thermal Behaviour of Trans-Poly(1-Octenylene). Die Makromol. Chemie 1988, 189, 2823–2837. [Google Scholar] [CrossRef]
- Calderon, N.; Ofstead, E.A.; Judy, W.A. Ring-Opening Polymerization of Unsaturated Alicyclic Compounds. J. Polym. Sci. Part A-1 Polym. Chem. 1967, 5, 2209–2217. [Google Scholar] [CrossRef]
- Shandryuk, G.A.; Denisova, Y.I.; Gringolts, M.L.; Krentsel, L.B.; Litmanovich, A.D.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Peculiarities of Crystallization in the Multiblock Copolymers of Norbornene and Cyclooctene. Eur. Polym. J. 2017, 86, 143–153. [Google Scholar] [CrossRef]
- Brostow, W.; Chiu, R.; Kalogeras, I.M.; Vassilikou-Dova, A. Prediction of Glass Transition Temperatures: Binary Blends and Copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- Denisova, Y.I.; Shandryuk, G.A.; Arinina, M.P.; Levin, I.S.; Zhigarev, V.A.; Gringolts, M.L.; Finkelshtein, E.S.; Malkin, A.Y.; Kudryavtsev, Y.V. Multiblock Copolymers of Norbornene and Cyclododecene: Chain Structure and Properties. Polymers 2021, 13, 1756. [Google Scholar] [CrossRef]
- Cruz-Morales, J.A.; Vargas, J.; Santiago, A.A.; Vásquez-García, S.R.; Tlenkopatchev, M.A.; de Lys, T.; López-González, M. Synthesis and Gas Transport Properties of New Polynorbornene Dicarboximides Bearing Trifluoromethyl Isomer Moieties. High Perform. Polym. 2016, 28, 1246–1262. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bixler, H.J. Flow of Gases through Polyethylene. J. Polym. Sci. 1961, 50, 413–439. [Google Scholar] [CrossRef]
- Cowling, R.; Park, G.S. Permeability, Solubility and Diffusion of Gases in Amorphous and Crystalline 1,4-Polybutadiene Membranes. J. Memb. Sci. 1979, 5, 199–207. [Google Scholar] [CrossRef]
- Starannikova, L.E.; Belov, N.A.; Shantorovich, V.P.; Suzuki, T.; Golenko, T.G.; Makovetskii, K.L.; Yampol’skii, Y.P. Transport and Physicochemical Parameters of Polypentenamer. Polym. Sci. Ser. A 2007, 49, 509–516. [Google Scholar] [CrossRef]
- Stern, S.A.; Shah, V.M.; Hardy, B.J. Structure-Permeability Relationships in Silicone Polymers. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1263–1298. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Z.-Y.; Sun, Z.Y. Self-assembly structures of amphiphilic multiblock copolymer in dilute solution. Soft Matter 2013, 9, 1947–1954. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, J.; Wang, L.; Xu, Z. Theoretical modeling and simulations of self-assembly of copolymersin solution. Prog. Polym. Sci. 2017, 75, 1–30. [Google Scholar] [CrossRef]





| Polymer | Cat | [mon]/ [cat], mol/mol | Time, h | c, mol·L−1 | Yield, % | Cis/trans % | Mw, kg·mol−1 | Ð (a) | |
|---|---|---|---|---|---|---|---|---|---|
| COE | NBF | ||||||||
| PCOE | G1 | 700:1 | 3 | 5 | 90 | 40/60 | - | 233 | 1.8 |
| G3 | 1000:1 | 3 | 5 | 93 | 13/87 | - | 401 | 1.9 | |
| PNBF (b) | G1 | 1460:1 | 24 | 0.8 | 96 | - | 29/71 | 385 | 1.6 |
| G3 | 270:1 | 1 | 0.5 | 85 | - | 45/55 | 86 | 1.5 | |
| PNBFD | G1 | 90:1 | 24 | 0.5 | 79 | - | 16/84 | 64 | 1.3 |
| G1 | 180:1 | 24 | 0.7 | 96 | - | 10/90 | 169 | 1.9 | |
| G3 | 290:1 | 1 | 0.6 | 87 | - | 52/48 | 256 | 1.1 | |
| PNBF/PCOE (c) | 1:3 mol/mol | 40/60 | 29/71 | 267 | 1.6 | ||||
| PNBFD/PCOE (d) | 1:3 mol/mol | 40/60 | 16/84 | 215 | 1.6 | ||||
| Polymer | Cat | [mon]/ [cat] (a), mol/mol | Feed Ratio (a), mol/mol | Time, h | c, mol·L−1 | Yield, % | Copol. Ratio (b), mol/mol | Cis/trans % | Mw, kg·mol−1 | Ð (c) | Mean Block Lengths | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COE | NBF(D) | LN | LCO | ||||||||||
| Random copolymers | |||||||||||||
| C1 | G1 | 650:1 | 1:1 | 1 | 3.6 | 93 | 1:1.2 | 41/59 | - | 256 | 1.8 | - | - |
| C2 | 850:1 | 1:3 | 1 | 5.8 | 92 | 1:3.5 | 40/60 | - | 347 | 2.0 | - | - | |
| C3 | 720:1 | 1:3 | 2 | 5.0 | 86 | 1:2.6 | 47/53 | 48/52 | 253 | 2.0 | - | - | |
| C3a | 660:1 | 1:2.3 | 2 | 4.6 | 82 | 1:2.1 | 53/47 | 49/51 | 226 | 1.6 | - | - | |
| C4 | 890:1 | 1:20 | 1 | 3.4 | 89 | 1:21.2 | 38/62 | - | 335 | 1.8 | - | - | |
| C5 | 700:1 | 1:1 | 1 | 2.2 | 87 | 1:1.2 | 42/58 | 30/70 | 679 | 2.9 | 1.2 | 1.6 | |
| C6 | 640:1 | 1:3 | 1 | 3.2 | 87 | 1:3.6 | 32/68 | 28/72 | 422 | 2.4 | 1.2 | 3.7 | |
| C7 | 830:1 | 1:20 | 1 | 3.8 | 91 | 1:36 | 38/62 | - | 306 | 1.8 | - | - | |
| Diblock copolymers | |||||||||||||
| D1 | G3 | 1100:1 | 1:3 | 1 | 1.6 | 91 | 1:3.2 | 20/80 | 45/55 | 409 | 1.9 | - | - |
| D2 | 1540:1 | 1:3 | 1 | 1.9 | 81 | 1:2.9 | 21/78 | 51/49 | 527 | 2.7 | - | - | |
| Multiblock copolymers | |||||||||||||
| M1 | G1 | 100:1 | 1:1 | 24 | 0.4 | 88 | 1:1 | 17/83 | 29/71 | 42 | 1.7 | - | - |
| M2 | G2 | 900:1 | 1:3 | 25 | 0.5 | 85 | 1:2.3 | 17/83 | 29/71 | 112 | 1.5 | - | - |
| M3 | G2 | 1100:1 | 1:3 | 24 | 0.5 | 73 | 1:2.6 | 23/77 | 27/73 | 80 | 1.5 | - | - |
| M4 | G2 | 810:1 | 1:3 | 24 | 0.5 | 79 | 1:2.6 | 21/79 | 16/84 | 190 | 1.8 | 39 | 92 |
| Polymer | Tg, °C | Tm, °C | −ΔH, J·g−1 | DC, % (a) | WCA, ° (b) | Polymer | Tg, °C | Tm, °C | −ΔH, J·g−1 | DC, % (a) | WCA, ° (b) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCOE (G1) | −81 | 35 | 45 | 41 | 97 | C1 | −8 | 31 | 1.8 | 6 | 102 |
| PCOE (G3) | −68 | 65 | 84 | 53 | n/a (c) | C2 | −69 | 21 | 18 | 30 | 104 |
| PNBF (G1) | 49 | - | - | - | 107 | C3 | −67 | 9 | 13 | 28 | n/a |
| PNBF (G3) | 57 | - | - | - | n/a | C3a | −75 | −5 | 9.0 | 25 | n/a |
| PNBFD (G1) (d) | 208 | - | - | - | 82 | C4 | - | 28 | 51 | 51 | n/a |
| PNBFD (G3) | 206 | - | - | - | 81 | C5 | 53 | - | - | - | 88 |
| PNBF/PCOE (d) | 53; −82 | 31 | 22 | 39 | 106 | C6 | 15 | - | - | - | 91 |
| PNBFD/PCOE (d) | 208; −78 | 28 | 26 | 47 | 96 | M1 | −69 | 65 | 21 | 53 | 105 |
| D1 | - | 60 | 37 | 48 | 102 | M2 | - | 63 | 35 | 51 | 103 |
| D2 | 210; −72 | 34 | 33 | 47 | 95 | M3 | - | 47 | 33 | 49 | n/a |
| M4 | 206; −54 | 35 | 20 | 30 | 92 |
| Gas | PNBF | C3 | C3a | D1 | PE (b) | PB1 (c) | PB2 (d) | PPM (e) | PDMS (f) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC (g) | Am (h) | SC (g) | Am (h) | SC (g) | Am (h) | |||||||
| He | 73 | 88 | 69 | 14 | 27 | 4.9 | 9 | n/a | n/a | n/a | 240 | 560 |
| H2 | 45 | 114 | 85 | 16 | 31 | n/a | n/a | 78 | 6.5 | 11 | 415 | n/a |
| O2 | 13 | 63 | 44 | 6.9 | 13 | 2.9 | 5.1 | n/a | n/a | n/a | 310 | 933 |
| N2 | 4.5 | 23 | 16 | 2.3 | 4.0 | 1.0 | 1.8 | 17 | 1.3 | 2.1 | 103 | 470 |
| CO2 | 54 | 323 | 234 | 34 | 65 | 13 | 23 | 394 | 20 | 33 | 2000 | 4553 |
| CH4 | 4.3 | 51 | 34 | 4.9 | 9.0 | 2.9 | 5.1 | n/a | n/a | n/a | 433 | 1353 |
| C2H6 | n/a | 120 | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 2070 | n/a |
| C3H8 | n/a | 232 | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 4910 | n/a |
| C4H10 | n/a | 453 (i) | 324 (i) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| n/a | 1751 (j) | 1246 (j) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 22810 (j) | n/a | |
| Gas | PNBF | C3 | C3a | D1 | PE (a) | PB1 (b) | PB2 (c) | PDMS (d) |
|---|---|---|---|---|---|---|---|---|
| O2 | 30 | 483 | 197 | 37 | 46 | n/a | n/a | n/a |
| N2 | 13 | 418 | 153 | 21 | 32 | 40 | 4.0 | n/a |
| CO2 | 15 | 331 | 123 | 22 | 37 | 30 | 3.8 | 2640 |
| CH4 | 6.9 | 210 | 79 | 13 | 19 | n/a | n/a | 2450 |
| C2H6 | n/a | 69 | 27 | n/a | n/a | n/a | n/a | n/a |
| C3H8 | n/a | 36 | 14 | n/a | n/a | n/a | n/a | n/a |
| C4H10 | n/a | 26 (e) | 10 (e) | n/a | n/a | n/a | n/a | n/a |
| n/a | 85 (f) | 31 (f) | n/a | n/a | n/a | n/a | n/a |
| Polymer | PNBF | C3 | C3a | D1 | PE (a) | PPM (b) |
|---|---|---|---|---|---|---|
| F, mol% | 31 | 10 | 12 | 9 | 0 | 0 |
| Gas pair | αP | |||||
| O2/N2 | 2.9 | 2.7 | 2.8 | 3.0 | 2.9 | 3.0 |
| He/H2 | 1.6 | 0.77 | 0.81 | 0.88 | n/a | 0.58 |
| N2/CH4 | 1.0 | 0.45 | 0.47 | 0.47 | 0.34 | 0.24 |
| Parameter | PNBFD | PCOE | C6 | M4 | D2 |
|---|---|---|---|---|---|
| (a) dn/dc, ×10−6 m3·kg−1 | 75 ± 1 | 61.5 ± 0.7 | 68.4 ± 0.1 | 68.1 ± 0.4 | 69.9 ± 0.3 |
| (b) Mw, kg·mol−1 | 266 ± 4 | 146 ± 15 | 238 ± 17 | 189 ± 6 | 385 ± 15 |
| (c) A2, ×10−4 mol·m3·kg−2 | 1.5 ± 0.3 | 24 ± 1 | 12.3 ± 0.6 | 3.5 ± 0.4 | 1.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adzhieva, O.A.; Gringolts, M.L.; Denisova, Y.I.; Shandryuk, G.A.; Litmanovich, E.A.; Nikiforov, R.Y.; Belov, N.A.; Kudryavtsev, Y.V. Effect of Chain Structure on the Various Properties of the Copolymers of Fluorinated Norbornenes with Cyclooctene. Polymers 2023, 15, 2157. https://doi.org/10.3390/polym15092157
Adzhieva OA, Gringolts ML, Denisova YI, Shandryuk GA, Litmanovich EA, Nikiforov RY, Belov NA, Kudryavtsev YV. Effect of Chain Structure on the Various Properties of the Copolymers of Fluorinated Norbornenes with Cyclooctene. Polymers. 2023; 15(9):2157. https://doi.org/10.3390/polym15092157
Chicago/Turabian StyleAdzhieva, Olga A., Maria L. Gringolts, Yulia I. Denisova, Georgiy A. Shandryuk, Ekaterina A. Litmanovich, Roman Yu. Nikiforov, Nikolay A. Belov, and Yaroslav V. Kudryavtsev. 2023. "Effect of Chain Structure on the Various Properties of the Copolymers of Fluorinated Norbornenes with Cyclooctene" Polymers 15, no. 9: 2157. https://doi.org/10.3390/polym15092157