Effects of Co-Solvent-Induced Self-Assembled Graphene-PVDF Composite Film on Piezoelectric Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixing Different Co-Solvents with PVDF
2.3. Fabrication of Graphene–PVDF Composite Film with Different Co-Solvent
2.4. Characterization of the Graphene–PVDF Composite Film with Different Co-Solvent
2.5. Piezoelectric Test of the Graphene–PVDF Composite Film with Different Co-Solvent
3. Results and Discussion
3.1. Photograph of Dispersion and Physical Characterization of Graphene–PVDF with Different Co-Solvent
3.2. Morphology of the Piezoelectric Nanogenerators Graphene–PVDF Composite Film
3.3. Chemical Characterization of the Graphene–PVDF Composite Film with Different Co-Solvent
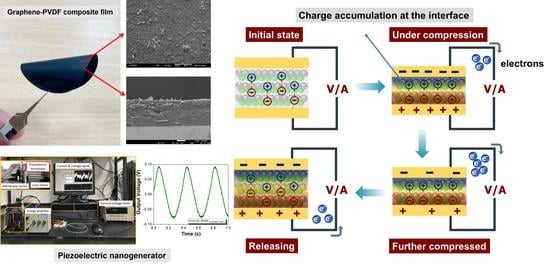
3.4. Piezoelectric Test of Graphene–PVDF Composite Film with Different Co-Solvent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karan, S.K.; Maiti, S.; Paria, S.; Maitra, A.; Si, S.K.; Kim, J.K.; Khatua, B.B. A new insight towards eggshell membrane as high energy conversion efficient bio-piezoelectric energy harvester. Mater. Today Energy 2018, 9, 114–125. [Google Scholar] [CrossRef]
- Bai, P.; Zhu, G.; Jing, Q.; Yang, J.; Chen, J.; Su, Y.; Ma, J.; Zhang, G.; Wang, Z.L. Membrane-based self-powered triboelectric sensors for pressure change detection and its uses in security surveillance and healthcare monitoring. Adv. Funct. Mater. 2014, 24, 5807–5813. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, X.; Oh, J.H. Fabrication of triboelectric nanogenerators based on electrospun polyimide nanofibers membrane. Sci. Rep. 2020, 10, 2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ericka, M.; Vasic, D.; Costa, F.; Poulin, G.; Tliba, S. Energy harvesting from vibration using a piezoelectric membrane. J. Phys. IV 2005, 128, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Lefeuvre, E.; Badel, A.; Benayad, A.; Lebrun, L.; Richard, C.; Guyomar, D. A comparison between several approaches of piezoelectric energy harvesting. J. Phys. IV 2005, 128, 177–186. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, C.; Niu, J.; Zhang, Y.; Li, C.; Hu, P. Coaxially aligned MWCNTs improve performance of electrospun P (VDF-TrFE)-based fibrous membrane applied in wearable piezoelectric nanogenerator. Compos. Part B Eng. 2019, 178, 107447. [Google Scholar] [CrossRef]
- Cao, X.; Xiong, Y.; Sun, J.; Zhu, X.; Sun, Q.; Wang, Z.L. Piezoelectric Nanogenerators Derived Self-Powered Sensors for Multifunctional Applications and Artificial Intelligence. Adv. Funct. Mater. 2021, 31, 2102983. [Google Scholar] [CrossRef]
- Subrahmanya, T.; Lin, P.T.; Chiao, Y.-H.; Widakdo, J.; Chuang, C.-H.; Rahmadhanty, S.F.; Yoshikawa, S.; Hung, W.-S. High performance self-heated membrane distillation system for energy efficient desalination process. J. Mater. Chem. A 2021, 9, 7868–7880. [Google Scholar]
- Stadlober, B.; Zirkl, M.; Irimia-Vladu, M. Route towards sustainable smart sensors: Ferroelectric polyvinylidene fluoride-based materials and their integration in flexible electronics. Chem. Soc. Rev. 2019, 48, 1787–1825. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.; Wang, T.; Yilmaz, D.E.; Van Duin, A.C. C/H/O/F/Al ReaxFF Force Field Development and Application to Study the Condensed-Phase Poly (vinylidene fluoride) and Reaction Mechanisms with Aluminum. J. Phys. Chem. C 2022, 126, 11058–11074. [Google Scholar] [CrossRef]
- Le Moel, A.; Duraud, J.; Balanzat, E. Modifications of polyvinylidene fluoride (PVDF) under high energy heavy ion, X-ray and electron irradiation studied by X-ray photoelectron spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1986, 18, 59–63. [Google Scholar] [CrossRef]
- Widakdo, J.; Austria, H.F.; Subrahmanya, T.; Suharyadi, E.; Hung, W.-S.; Wang, C.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Switching gas permeation through smart membranes by external stimuli: A review. J. Mater. Chem. A 2022, 10, 16743. [Google Scholar] [CrossRef]
- Widakdo, J.; Chiao, Y.-H.; Lai, Y.-L.; Imawan, A.C.; Wang, F.-M.; Hung, W.-S. Mechanism of a self-assembling smart and electrically responsive PVDF–graphene membrane for controlled gas separation. ACS Appl. Mater. Interfaces 2020, 12, 30915–30924. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanya, T.; Arshad, A.B.; Lin, P.T.; Widakdo, J.; Makari, H.; Austria, H.F.M.; Hu, C.-C.; Lai, J.-Y.; Hung, W.-S. A review of recent progress in polymeric electrospun nanofiber membranes in addressing safe water global issues. RSC Adv. 2021, 11, 9638–9663. [Google Scholar]
- Gregorio, R.; Ueno, E. Effect of crystalline phase, orientation and temperature on the dielectric properties of poly (vinylidene fluoride) (PVDF). J. Mater. Sci. 1999, 34, 4489–4500. [Google Scholar] [CrossRef]
- Widakdo, J.; Huang, T.-H.; Subrahmanya, T.; Austria, H.F.M.; Hung, W.-S.; Wang, C.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Tailoring of graphene–organic frameworks membrane to enable reversed electrical-switchable permselectivity in CO2 separation. Carbon 2021, 182, 545–558. [Google Scholar] [CrossRef]
- An, N.; Liu, S.; Fang, C.; Yu, R.; Zhou, X.; Cheng, Y. Preparation and properties of β-phase graphene oxide/PVDF composite films. J. Appl. Polym. Sci. 2015, 132, 41577. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Zhou, Y.; Qu, Z.; Wang, H.; Zhang, Y.; Zhou, H.; Yan, C. Facile preparation of highly oriented poly (vinylidene fluoride) uniform films and their ferro-and piezoelectric properties. RSC Adv. 2017, 7, 17038–17043. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Shao, H.-Q.; Liu, Y.; Tang, C.-Y.; Zhao, X.; Ke, K.; Bao, R.-Y.; Yang, M.-B.; Yang, W. Boosting piezoelectric response of PVDF-TrFE via MXene for self-powered linear pressure sensor. Compos. Sci. Technol. 2021, 202, 108600. [Google Scholar] [CrossRef]
- Kabir, E.; Khatun, M.; Nasrin, L.; Raihan, M.J.; Rahman, M. Pure β-phase formation in polyvinylidene fluoride (PVDF)-carbon nanotube composites. J. Phys. D Appl. Phys. 2017, 50, 163002. [Google Scholar] [CrossRef]
- Kaeopisan, A.; Wattanasarn, H. Piezoelectric PVDF/CNT Flexible Applied on Motorcycle. Integr. Ferroelectr. 2021, 214, 166–172. [Google Scholar] [CrossRef]
- Mahmud, M.; Adhikary, P.; Zolfagharian, A.; Adams, S.; Kaynak, A.; Kouzani, A.Z. Advanced Design, Fabrication, and Applications of 3D-Printable Piezoelectric Nanogenerators. Electron. Mater. Lett. 2022, 18, 129–144. [Google Scholar] [CrossRef]
- Jang, W.; Hou, J.; Byun, H.; Lee, J.Y. Preparation of PVdF/Fe3O4-GO (MGO) Composite Membrane by Using Electrospinning Technology and its Arsenic Removal Characteristics. Membr. J. 2016, 26, 480–489. [Google Scholar] [CrossRef]
- Widakdo, J.; Huang, T.-J.; Subrahmanya, T.; Austria, H.F.M.; Chou, H.-L.; Hung, W.-S.; Wang, C.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Bioinspired ionic liquid-graphene based smart membranes with electrical tunable channels for gas separation. Appl. Mater. Today 2022, 27, 101441. [Google Scholar] [CrossRef]
- Austria, H.F.; Subrahmanya, T.; Setiawan, O.; Widakdo, J.; Chiao, Y.-H.; Hung, W.-S.; Wang, C.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. A review on the recent advancements in graphene-based membranes and their applications as stimuli-responsive separation materials. J. Mater. Chem. A 2021, 9, 21510–21531. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, J.X.; Chiao, Y.H.; Chang, C.M.; Hung, W.S.; Lue, S.J.; Wang, C.F.; Hu, C.C.; Lee, K.R.; Pan, H.H. Tailoring of a Piezo-Photo-Thermal Solar Evaporator for Simultaneous Steam and Power Generation. Adv. Funct. Mater. 2021, 31, 2010422. [Google Scholar] [CrossRef]
- Huang, L.; Lu, C.; Wang, F.; Dong, X. Piezoelectric property of PVDF/graphene composite films using 1H, 1H, 2H, 2H-Perfluorooctyltriethoxysilane as a modifying agent. J. Alloy. Compd. 2016, 688, 885–892. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Wei, W.; Qu, X. Extraordinary physical properties of functionalized graphene. Small 2012, 8, 2138–2151. [Google Scholar] [CrossRef]
- Jang, H.; Park, Y.J.; Chen, X.; Das, T.; Kim, M.S.; Ahn, J.H. Graphene-based flexible and stretchable electronics. Adv. Mater. 2016, 28, 4184–4202. [Google Scholar] [CrossRef]
- Grande, L.; Chundi, V.T.; Wei, D.; Bower, C.; Andrew, P.; Ryhaenen, T. Graphene for energy harvesting/storage devices and printed electronics. Particuology 2012, 10, 1–8. [Google Scholar] [CrossRef]
- Athanasekou, C.; Sapalidis, A.; Katris, I.; Savopoulou, E.; Beltsios, K.; Tsoufis, T.; Kaltzoglou, A.; Falaras, P.; Bounos, G.; Antoniou, M. Mixed Matrix PVDF/Graphene and Composite-Skin PVDF/Graphene Oxide Membranes Applied in Membrane Distillation. Polym. Eng. Sci. 2019, 59, E262–E278. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, B.; Wang, L.; Zhang, Z.; Zhang, H.; Zhao, X.; Guo, X. Hydrophobic PVDF/graphene hybrid membrane for CO2 absorption in membrane contactor. J. Membr. Sci. 2016, 520, 120–129. [Google Scholar] [CrossRef]
- Khosravi, M.; Seyfi, J.; Saeidi, A.; Khonakdar, H.A. Spin-coated polyvinylidene fluoride/graphene nanocomposite thin films with improved β-phase content and electrical conductivity. J. Mater. Sci. 2020, 55, 6696–6707. [Google Scholar] [CrossRef]
- Sigamani, N.; Ounaies, Z.; Ehlert, G.; Sodano, H. Preparation of reduced graphene/PVDF nanocomposites using co-solvent approach. In Behavior and Mechanics of Multifunctional Materials and Composites, Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, San Diego, CA, USA, 28 March 2012; SPIE: Bellingham, WA, USA, 2012. [Google Scholar]
- Oflaz, K.; Özaytekin, İ. Analysis of electrospinning and additive effect on β phase content of electrospun PVDF nanofiber mats for piezoelectric energy harvester nanogenerators. Smart Mater. Struct. 2022, 31, 105022. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Li, L. Fabrication of PVDF/graphene composites with enhanced β phase via conventional melt processing assisted by solid state shear milling technology. RSC Adv. 2020, 10, 3391–3401. [Google Scholar] [CrossRef] [Green Version]
- Sencadas, V.; Gregorio, R., Jr.; Lanceros-Méndez, S. α to β phase transformation and microestructural changes of PVDF films induced by uniaxial stretch. J. Macromol. Sci. 2009, 48, 514–525. [Google Scholar] [CrossRef]
- Brunengo, E.; Luciano, G.; Canu, G.; Canetti, M.; Conzatti, L.; Castellano, M.; Stagnaro, P. Double-step moulding: An effective method to induce the formation of β-phase in PVDF. Polymer 2020, 19, 122345. [Google Scholar] [CrossRef]
- Habibur, R.M.; Yaqoob, U.; Muhammad, S.; Uddin, A.I.; Kim, H.C. The effect of RGO on dielectric and energy harvesting properties of P (VDF-TrFE) matrix by optimizing electroactive β phase without traditional polling process. Mater. Chem. Phys. 2018, 215, 46–55. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Wang, X. Formation of poly (vinylidene fluoride) crystalline phases from tetrahydrofuran/N, N-dimethylformamide mixed solvent. J. Mater. Sci. 2008, 43, 398–401. [Google Scholar] [CrossRef]
- Salem, M.S.A.; El-Shazly, A.H.; El-Marghany, M.R.; Sabry, M.N.; Nady, N. Effect of adding functionalized graphene on the performance of PVDF membrane in direct contact membrane distillation. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2019. [Google Scholar]
- Zha, D.-A.; Mei, S.; Wang, Z.; Li, H.; Shi, Z.; Jin, Z. Superhydrophobic polyvinylidene fluoride/graphene porous materials. Carbon 2011, 49, 5166–5172. [Google Scholar] [CrossRef]
- Subrahmanya, T.; Widakdo, J.; Austria, H.F.M.; Hung, W.-S.; Kurkuri, M.D.; Wang, C.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Flow-through in-situ evaporation membrane enabled self-heated membrane distillation for efficient desalination of hypersaline water. Chem. Eng. J. 2023, 452, 139170. [Google Scholar]
- Greenlee, L.F.; Rentz, N.S. Influence of nanoparticle processing and additives on PES casting solution viscosity and cast membrane characteristics. Polymer 2016, 103, 498–508. [Google Scholar] [CrossRef]
- Pérez, L.A.; Bajales, N.; Lacconi, G.I. Raman spectroscopy coupled with AFM scan head: A versatile combination for tailoring graphene oxide/reduced graphene oxide hybrid materials. Appl. Surf. Sci. 2019, 495, 143539. [Google Scholar] [CrossRef]
- Li, J.-W.; Huang, C.-Y.; Chen, K.-Y.; Chen, J.-X.; Hsu, X.-Y.; Chen, Y.-F.; Kuo, C.-F.J.; Cheng, C.-C.; Suen, M.-C.; Chiu, C.-W. Enhanced piezoelectric properties of poly (vinylidenefluoride-co-trifluoroethylene)/carbon-based nanomaterial composite films for pressure sensing applications. Polymers 2020, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, H.; Noormohammadi, M.; Kashi, M.A.; Amiri, M.H.; Michels, J.J.; Asadi, K.; Abolhasani, M.M. Self-Poled Sausage-Like PVDF Nanowires Produced by Confined Phase Inversion as Novel Piezoelectric Nanogenerators. Adv. Mater. Interfaces 2021, 8, 2001734. [Google Scholar] [CrossRef]
- Potrzebowska, N.; Cavani, O.; Oral, O.; Doaré, O.; Melilli, G.; Wegrowe, J.-E.; Clochard, M.-C. Mixing nanostructured Ni/piezoPVDF composite thin films with e-beam irradiation: A beneficial synergy to piezoelectric response. Mater. Today Commun. 2021, 28, 102528. [Google Scholar] [CrossRef]
- Samadi, A.; Ahmadi, R.; Hosseini, S.M. Influence of TiO2-Fe3O4-MWCNT hybrid nanotubes on piezoelectric and electromagnetic wave absorption properties of electrospun PVDF nanocomposites. Org. Electron. 2019, 75, 105405. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; AlMaadeed, M.A.A. Investigation on the effect of γ-irradiation on the dielectric and piezoelectric properties of stretchable PVDF/Fe–ZnO nanocomposites for self-powering devices. Soft Matter. 2018, 14, 8803–8813. [Google Scholar] [CrossRef]
- Jella, V.; Ippili, S.; Eom, J.-H.; Choi, J.; Yoon, S.-G. Enhanced output performance of a flexible piezoelectric energy harvester based on stable MAPbI3-PVDF composite films. Nano Energy 2018, 53, 46–56. [Google Scholar] [CrossRef]








| Sample Name | Fabrication | Output Voltage (V) | Reference |
|---|---|---|---|
| PVDF-TrFE | Mixing | 0.71 | [47] |
| CNT–COOH/PVDF-T 1 wt% | 1.22 | ||
| CNT–COOH:SMA EF40-M/PVDF-T 1:1 | 1.34 | ||
| PVDF S.NWs + AAO | Electrospinning | 1.97 | [48] |
| Ni NWs/PVDF | Electrospinning | 1.75 | [49] |
| PVDF/TiO2–Fe3O4–MWCNT | Electrospinning | 51.42 mV/N | [50] |
| Fe–ZnO/PVDF | Gamma irradiation of casted films | 2.4 | [51] |
| Gr-PVDFACE/NMP 30 wt% | Mixing | 0.092 | This work |
| Gr-PVDFTHF/NMP 20 wt% | Mixing | 0.091 | |
| Gr-PVDFwater/NMP 40 wt% | Mixing | 0.051 | |
| Gr-PVDFEtOH/NMP 20 wt% | Mixing | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widakdo, J.; Lei, W.-C.; Anawati, A.; Thagare Manjunatha, S.; Austria, H.F.M.; Setiawan, O.; Huang, T.-H.; Chiao, Y.-H.; Hung, W.-S.; Ho, M.-H. Effects of Co-Solvent-Induced Self-Assembled Graphene-PVDF Composite Film on Piezoelectric Application. Polymers 2023, 15, 137. https://doi.org/10.3390/polym15010137
Widakdo J, Lei W-C, Anawati A, Thagare Manjunatha S, Austria HFM, Setiawan O, Huang T-H, Chiao Y-H, Hung W-S, Ho M-H. Effects of Co-Solvent-Induced Self-Assembled Graphene-PVDF Composite Film on Piezoelectric Application. Polymers. 2023; 15(1):137. https://doi.org/10.3390/polym15010137
Chicago/Turabian StyleWidakdo, Januar, Wen-Ching Lei, Anawati Anawati, Subrahmanya Thagare Manjunatha, Hannah Faye M. Austria, Owen Setiawan, Tsung-Han Huang, Yu-Hsuan Chiao, Wei-Song Hung, and Ming-Hua Ho. 2023. "Effects of Co-Solvent-Induced Self-Assembled Graphene-PVDF Composite Film on Piezoelectric Application" Polymers 15, no. 1: 137. https://doi.org/10.3390/polym15010137