Variations in Microstructural and Physicochemical Properties of Soy Wax/Soybean Oil-Derived Oleogels Using Soy Lecithin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
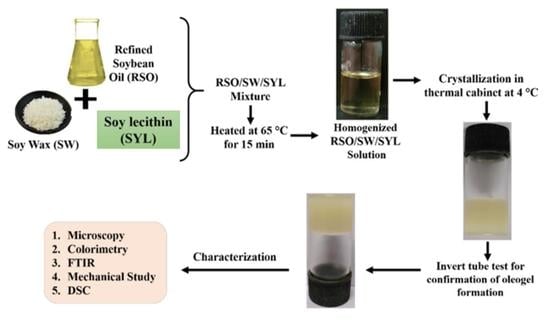
2.2. Methods
2.3. Microscopic Study
2.4. Colorimetric Analysis
2.5. FTIR Analysis
2.6. Mechanical Study
2.7. DSC
2.8. Statistical Analysis
3. Results
3.1. Microscopic Study
3.2. Colorimetric Analysis
3.3. FTIR Analysis
3.4. Mechanical Studies
3.5. DSC Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cesàro, A.; Bellich, B.; Borgogna, M. Biophysical functionality in polysaccharides: From Lego-blocks to nano-particles. Eur. Biophys. J. 2012, 41, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Jarzębski, M.; Bellich, B.; Białopiotrowicz, T.; Śliwa, T.; Kościński, J.; Cesàro, A. Particle tracking analysis in food and hydrocolloids investigations. Food Hydrocoll. 2017, 68, 90–101. [Google Scholar] [CrossRef]
- Shah, P. Polymers in food. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 567–592. [Google Scholar]
- Sidor, A.; Rzymski, P. Dietary choices and habits during COVID-19 lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Czarniecka-Skubina, E.; Pielak, M.; Sałek, P.; Głuchowski, A.; Kobus-Cisowska, J.; Owczarek, T. Use of food services by consumers in the SARS-CoV-2 pandemic. How the eating habits of consumers changed in view of the new disease risk factors? Nutrients 2021, 13, 2760. [Google Scholar] [CrossRef]
- Bhat, S.; Maganja, D.; Huang, L.; Wu, J.H.; Marklund, M. Influence of Heating during Cooking on Trans Fatty Acid Content of Edible Oils: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1489. [Google Scholar] [CrossRef]
- Estadella, D.; da Penha Oller do Nascimento, C.; Oyama, L.M.; Ribeiro, E.B.; Damaso, A.R.; de Piano, A. Lipotoxicity: Effects of dietary saturated and transfatty acids. Mediat. Inflamm. 2013, 2013, 137579. [Google Scholar] [CrossRef]
- Astrup, A.; Bertram, H.; Bonjor, J.-P.; De Groot, L.; De Oliviera Otto, M.; Feeney, E.; Garg, M.L.; Givens, I.; Kok, F.J.; Krauss, R.M.; et al. WHO draft guidelines on dietary saturated and trans fatty acids: Time for a new approach? BMJ 2019, 366, 4137. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur. J. Pediatrics 2010, 169, 149–164. [Google Scholar] [CrossRef]
- Zambiazi, R.C.; Przybylski, R.; Zambiazi, M.W.; Mendonça, C.B. Fatty acid composition of vegetable oils and fats. Bol. Cent. Pesqui. Processamento Aliment. 2007, 25. [Google Scholar]
- Yılmaz, E.; Öğütcü, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct. 2015, 6, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Hwang, H.-S. A critical review on structures, health effects, oxidative stability, and sensory properties of oleogels. Biocatal. Agric. Biotechnol. 2020, 26, 101657. [Google Scholar] [CrossRef]
- Scharfe, M.; Ahmane, Y.; Seilert, J.; Keim, J.; Flöter, E. On the effect of minor oil components on β-sitosterol/γ-oryzanol oleogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1800487. [Google Scholar] [CrossRef]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative study on the ability of different oleogelators to structure sunflower oil. Food Biophys. 2020, 15, 42–49. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinick, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef]
- Dassanayake LS, K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical properties of rice bran wax in bulk and organogels. J. Am. Oil Chem. Soc. 2009, 86, 1163–1173. [Google Scholar] [CrossRef]
- Yi, B.; Kim, M.J.; Lee, S.Y.; Lee, J. Physicochemical properties and oxidative stability of oleogels made of carnauba wax with canola oil or beeswax with grapeseed oil. Food Sci. Biotechnol. 2017, 26, 79–87. [Google Scholar] [CrossRef]
- Saralaya, S.; Jayanth, B.S.; Thomas, N.S.; Sunil, S.M. Bee wax and honey—a primer for OMFS. Oral Maxillofac. Surg. 2021, 25, 1–6. [Google Scholar] [CrossRef]
- Trisnadewi, T.; Kusrini, E.; Nurjaya, D.M.; Putra, N.; Mahlia, T.M.I. Experimental analysis of natural wax as phase change material by thermal cycling test using thermoelectric system. J. Energy Storage 2021, 40, 102703. [Google Scholar] [CrossRef]
- Yao, L.; Lio, J.; Wang, T.; Jarboe, D.H. Synthesis and characterization of acetylated and stearylyzed soy wax. J. Am. Oil Chem. Soc. 2013, 90, 1063–1071. [Google Scholar] [CrossRef]
- Yao, L.; Wang, T. Textural and physical properties of biorenewable “waxes” containing partial acylglycerides. J. Am. Oil Chem. Soc. 2012, 89, 155–166. [Google Scholar] [CrossRef]
- Khosla, P.; Sundram, K. A Supplement on Palm Oil–Why? Am. Oil Chem. Soc. 2010, 29, 237S–239S. [Google Scholar] [CrossRef] [PubMed]
- Staughton, J. The remarkable benefits of soya bean oil. Oilseeds Focus 2020, 6, 31. [Google Scholar]
- Van Nieuwenhuyzen, W.; Tomás, M.C. Update on vegetable lecithin and phospholipid technologies. Eur. J. Lipid Sci. Technol. 2008, 110, 472–486. [Google Scholar] [CrossRef]
- List, G.R. Soybean lecithin: Food, industrial uses, and other applications. Polar Lipids 2015, 1–33. [Google Scholar] [CrossRef]
- Arima, S.; Ueji, T.; Ueno, S.; Ogawa, A.; Sato, K. Retardation of crystallization-induced destabilization of PMF-in-water emulsion with emulsifier additives. Colloids Surf. B Biointerfaces 2007, 55, 98–106. [Google Scholar] [CrossRef]
- Petkowicz, C.L.; Williams, P.A. Pectins from food waste: Characterization and functional properties of a pectin extracted from broccoli stalk. Food Hydrocoll. 2020, 107, 105930. [Google Scholar] [CrossRef]
- Chen, R.; Williams, P.A.; Shu, J.; Luo, S.; Chen, J.; Liu, C. Pectin adsorption onto and penetration into starch granules and the effect on the gelatinization process and rheological properties. Food Hydrocoll. 2022, 129, 107618. [Google Scholar] [CrossRef]
- Zhao, C.; Yin, H.; Yan, J.; Niu, X.; Qi, B.; Liu, J. Structure and acid-induced gelation properties of soy protein isolate–maltodextrin glycation conjugates with ultrasonic pretreatment. Food Hydrocoll. 2021, 112, 106278. [Google Scholar] [CrossRef]
- Paul, S.R.; Qureshi, D.; Yogalakshmi, Y.; Nayak, S.K.; Singh, V.K.; Syed, I.; Sarkar, P.; Pal, K. Development of bigels based on stearic acid–rice bran oil oleogels and tamarind gum hydrogels for controlled delivery applications. J. Surfactants Deterg. 2018, 21, 17–29. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil–Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Sagiri, S.S.; Singh, V.K.; Pal, K.; Banerjee, I.; Basak, P. Stearic acid based oleogels: A study on the molecular, thermal and mechanical properties. Mater. Sci. Eng. C 2015, 48, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake LS, K.; Kodali, D.R.; Ueno, S. Formation of oleogels based on edible lipid materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 432–439. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Moschakis, T.; Biliaderis, C.G.; Lazaridou, A.; Katsanidis, E. Crystalline microstructure and physicochemical properties of olive oil oleogels formulated with monoglycerides and phytosterols. LWT 2022, 154, 112815. [Google Scholar] [CrossRef]
- Pang, M.; Cao, L.; Kang, S.; Jiang, S.; Cao, L. Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides. Foods 2021, 10, 1828. [Google Scholar] [CrossRef] [PubMed]
- Pernetti, M.; van Malssen, K.; Kalnin, D.; Flöter, E. Structuring edible oil with lecithin and sorbitan tri-stearate. Food Hydrocoll. 2007, 21, 855–861. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Scholten, E. Self-assemblies of lecithin and α-tocopherol as gelators of lipid material. RSC Adv. 2014, 4, 2466–2473. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Sterol-based oleogels2019 characterization envisioning food applications. J. Sci. Food Agric. 2019, 99, 3318–3325. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Smith, D.E. Color analysis. In Food Analysis; Springer: Cham, Switzerland, 2017; pp. 545–555. [Google Scholar]
- Hiamey, S.E.; Amenumey, E.K.; Mensah, I. Critical success factors for food tourism destinations: A socio-cultural perspective. Int. J. Tour. Res. 2021, 23, 192–205. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M. Comparative analysis of olive oil organogels containing beeswax and sunflower wax with breakfast margarine. J. Food Sci. 2014, 79, E1732–E1738. [Google Scholar] [CrossRef] [PubMed]
- Whetzel, N. Selecting Yellowness and Whiteness Indices to Measure Samples—AN-1009. 2019. Available online: https://support.hunterlab.com/hc/en-us/articles/204138095-Selecting-Yellowness-and-Whiteness-Indices-to-Measure-Samples-AN-1009 (accessed on 23 August 2019).
- Pathare, P.B.; Opara, U.L.; Al-Said FA, J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Muste, S.J.P. Application of Analytical Methods for the Comprehensive Analysis of Oleogels—A Review. Polymers 2021, 13, 1934. [Google Scholar] [CrossRef]
- Philips, K. CIELAB Color Space. HunterLab. 2020. Available online: https://blog.hunterlab.com/blog/uncategorized/cielab-color-space/ (accessed on 3 September 2020).
- Mendoza, F.; Dejmek, P.; Aguilera, J.M. Calibrated color measurements of agricultural foods using image analysis. Postharvest Biol. Technol. 2006, 41, 285–295. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Merchán Sandoval, J.; Carelli, A.; Palla, C.; Baümler, E. Preparation and characterization of oleogel emulsions: A comparative study between the use of recovered and commercial sunflower waxes as structuring agent. J. Food Sci. 2020, 85, 2866–2878. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Kamyabi, A.; Shahalizade, T.; Asadollahi Taheri, H. Preparation of highly flexible cellulose acetate membranes modified by hyperbranched poly(amine ester)-epoxidized soybean oil and evaluation of its filtration properties. Cellulose 2017, 24, 5389–5402. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, B.; Kaur, A.; Singh, N. Chemical, thermal, rheological and FTIR studies of vegetable oils and their effect on eggless muffin characteristics. J. Food Process. Preserv. 2019, 43, e13978. [Google Scholar] [CrossRef]
- Sim, S.F.; Ting, W. An automated approach for analysis of Fourier Transform Infrared (FTIR) spectra of edible oils. Talanta 2012, 88, 537–543. [Google Scholar] [CrossRef]
- Alshuiael, S.M.; Al-Ghouti, M.A. Multivariate analysis for FTIR in understanding treatment of used cooking oil using activated carbon prepared from olive stone. PLoS ONE 2020, 15, e0232997. [Google Scholar] [CrossRef]
- Trujillo-Ramírez, D.; Reyes, I.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Chia seed oil-candelilla wax oleogels structural features and viscoelasticity are enhanced by annealing. LWT 2022, 153, 112433. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B.; Hashim, P.; Ismail, A. FTIR spectroscopy combined with chemometrics for analysis of lard adulteration in some vegetable oils Espectroscopia FTIR combinada con quimiometría para el análisis de adulteración con grasa de cerdo de aceites vegetales. CyTA J. Food 2011, 9, 96–101. [Google Scholar] [CrossRef]
- Wu, Y. Soybean Lecithin Composition, Fractionation, and Functionality; Iowa State University: Ames, IA, USA, 2002. [Google Scholar]
- Pires, L.N.; Brandão, G.C.; Teixeira, L.S.G. Determination of phospholipids in soybean lecithin samples via the phosphorus monoxide molecule by high-resolution continuum source graphite furnace molecular absorption spectrometry. Food Chem. 2017, 225, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Quintás, G.; Esteve-Turrillas, F.A.; Garrigues, S.; De la Guardia, M. On-line gel permeation chromatography–attenuated total reflectance–Fourier transform infrared determination of lecithin and soybean oil in dietary supplements. J. Chromatogr. A 2008, 1185, 71–77. [Google Scholar] [CrossRef]
- Hoffmann, D.J.; Burr, M.T.; Kroll, M.J.; Logan, L.M. Characterization of Flaring and Non-Flaring Container Filled Votive Candles. Fire Technol. 2014, 50, 1379–1389. [Google Scholar] [CrossRef]
- Parish, E.J.; Boos, T.L.; Li, S. The Chemistry of Waxes and Sterols. CRC Press: Boka Raton, FL, USA, 2002; pp. 103–132. [Google Scholar]
- Swe, M.T.H.; Asavapichayont, P. Effect of silicone oil on the microstructure, gelation and rheological properties of sorbitan monostearate–sesame oil oleogels. Asian J. Pharm. Sci. 2018, 13, 485–497. [Google Scholar] [CrossRef]
- Yamaue, T.; Doi, M. The stress diffusion coupling in the swelling dynamics of cylindrical gels. J. Chem. Phys. 2005, 122, 084703. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Kasiviswanathan, U.; Shaw, G.S.; Singh, M.; Anis, A.; Pal, K. Effect of sorbitan monostearate concentration on the thermal, mechanical and drug release properties of oleogels. Korean J. Chem. Eng. 2016, 33, 1720–1727. [Google Scholar] [CrossRef]
- Uvanesh, K.; Sagiri, S.S.; Senthilguru, K.; Pramanik, K.; Banerjee, I.; Anis, A.; Al-Zahrani, S.M.; Pal, K. Effect of span 60 on the microstructure, crystallization kinetics, and mechanical properties of stearic acid oleogels: An in-depth analysis. J. Food Sci. 2016, 81, E380–E387. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Jacewicz, D.; Sielicka, A.; Chmurzyński, L. Characterization of polymers based on differential scanning calorimetry based techniques. Trends Anal. Chem. 2019, 110, 51–56. [Google Scholar] [CrossRef]
- Blake, A.I.; Toro-Vazquez, J.F.; Hwang, H.-S. Chapter 6–Wax Oleogels. In Edible Oleogels, 2nd ed.; Marangoni, A.G., Garti, N., Eds.; AOCS Press: Urbana, IL, USA, 2018; pp. 133–171. [Google Scholar]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.; Cunha, R.L. Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Okuro, P.K.; Tavernier, I.; Sintang MD, B.; Skirtach, A.G.; Vicente, A.A.; Dewettinck, K.; Cunha, R.L. Synergistic interactions between lecithin and fruit wax in oleogel formation. Food Funct. 2018, 9, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhuang, K.; Lin, S.; Zhang, Z.; Li, X. Determination of Supercooling Degree, Nucleation and Growth Rates, and Particle Size for Ice Slurry Crystallization in Vacuum. Crystals 2017, 7, 128. [Google Scholar] [CrossRef]
- Uvanesh, K.; Sagiri, S.S.; Banerjee, I.; Shaikh, H.; Pramanik, K.; Anis, A.; Pal, K. Effect of Tween 20 on the Properties of Stearate Oleogels: An in-Depth Analysis. J. Am. Oil Chem. Soc. 2016, 93, 711–719. [Google Scholar] [CrossRef]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef]
- Temkov, M.; Mureșan, V. Tailoring the structure of lipids, oleogels and fat replacers by different approaches for solving the trans-fat issue—A review. Foods 2021, 10, 1376. [Google Scholar] [CrossRef]







| SAMPLES | RSO (g) | SW (g) | SYL Stock (g) |
|---|---|---|---|
| SE0 | 35.60 | 4.40 | 0.00 |
| SE1 | 34.60 | 4.40 | 1.00 |
| SE3 | 32.60 | 4.40 | 3.00 |
| SE5 | 30.60 | 4.40 | 5.00 |
| SE10 | 25.60 | 4.40 | 10.00 |
| Samples | Tm (°C) | ΔHm (mW/mg) | Tc (°C) | ΔHc (mW/mg) | Supercooling (ΔT) |
|---|---|---|---|---|---|
| SE0 | 42.97 | 0.43 | 22.11 | −0.24 | 20.86 |
| SE1 | 42.40 | 0.38 | 22.12 | −0.22 | 20.28 |
| SE3 | 41.60 | 0.32 | 22.11 | −0.20 | 19.49 |
| SE5 | 42.87 | 0.38 | 22.12 | −0.26 | 20.75 |
| SE10 | 42.70 | 0.33 | 22.12 | −0.23 | 20.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sena, B.; Dhal, S.; Sahu, D.; Sarkar, P.; Mohanty, B.; Jarzębski, M.; Wieruszewski, M.; Behera, H.; Pal, K. Variations in Microstructural and Physicochemical Properties of Soy Wax/Soybean Oil-Derived Oleogels Using Soy Lecithin. Polymers 2022, 14, 3928. https://doi.org/10.3390/polym14193928
Sena B, Dhal S, Sahu D, Sarkar P, Mohanty B, Jarzębski M, Wieruszewski M, Behera H, Pal K. Variations in Microstructural and Physicochemical Properties of Soy Wax/Soybean Oil-Derived Oleogels Using Soy Lecithin. Polymers. 2022; 14(19):3928. https://doi.org/10.3390/polym14193928
Chicago/Turabian StyleSena, Biswajit, Somali Dhal, Deblu Sahu, Preetam Sarkar, Biswaranjan Mohanty, Maciej Jarzębski, Marek Wieruszewski, Haladhar Behera, and Kunal Pal. 2022. "Variations in Microstructural and Physicochemical Properties of Soy Wax/Soybean Oil-Derived Oleogels Using Soy Lecithin" Polymers 14, no. 19: 3928. https://doi.org/10.3390/polym14193928