Shape Memory Polymer-Based Endovascular Devices: Design Criteria and Future Perspective
Abstract
:1. Introduction
2. Current Endovascular Devices and Limitations
2.1. Generalities of Endovascular Therapies
2.2. Hydrogel-Coated Coils
2.3. Hydrogel/Liquid Embolic Materials
2.4. Woven EndoBridge (WEB)
2.5. Flow Diversion Devices (Flow Diverters)
2.6. TrelliX
2.7. Summary of Current Endovascular Devices
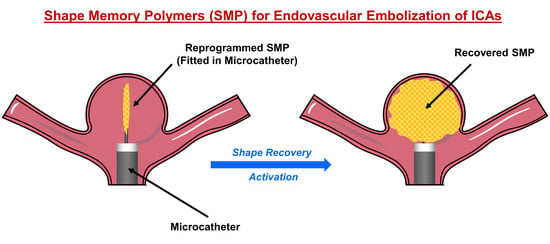
3. Shape Memory Polymers for Endovascular Embolization of ICAs
3.1. Polymer Architecture
3.1.1. Physically Crosslinked Co-Polymers
3.1.2. Covalently Crosslinked Copolymers
3.2. Porous Shape Memory Polymers
3.2.1. Gas Foaming
3.2.2. Particle Leaching
3.2.3. Additive Manufacturing (3D Printing)
3.3. Actuators for SMPs
3.3.1. Joule Heating Activation
3.3.2. Infrared (IR) Light Activation
3.3.3. Electromagnetic Activation
3.3.4. pH Activation
4. Potential of SMPs for ICA Endovascular Therapies and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | three dimensions |
| BA | butylacrylate |
| CNT | carbon nanotube |
| DMSO | dimethyl sulfoxide |
| FDM | fused deposition modeling |
| FRID | Flow-Redirection Endoluminal Device |
| GDC | Guglielmi detachable coil |
| GREAT | German–French Randomized Endovascular Aneurysm Trial |
| HELPS | HydroCoil endovascular aneurysm occlusion and packing study |
| ICA | intracranial aneurysm |
| IR | infrared |
| ISAT | International Subarachnoid Aneurysm Trial |
| PCO | polycyclooctene |
| PCL | polycaprolactone |
| PEG | polyethylene glycol |
| PEGDMA | polyethylene glycol dimethacrylate |
| PLA | polylactic acid |
| PPy | polypyrrole |
| PRET | Patients Prone to Recurrence After Endovascular Treatment Trial |
| PU | polyurethane |
| PVA | polyvinyl alcohol |
| RROC | Raymond–Roy occlusion classification |
| SMP | shape memory polymer |
| UV | ultraviolet |
| WEB | woven EndoBridge |
| WNBIAs | wide-necked bifurcation intracranial aneurysms |
References
- Toth, G.; Cerejo, R. Intracranial aneurysms: Review of current science and management. Vasc. Med. 2018, 23, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I. Intracranial aneurysms. N. Engl. J. Med. 1997, 336, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Keedy, A. An overview of intracranial aneurysms. McGill J. Med. 2006, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Rinkel, G.J. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 2016, 12, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Greving, J.P.; Wermer, M.J.; Brown, R.D.J.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Johnston, S.C.; Dudley, R.A.; Gress, D.R.; Ono, L. Surgical and endovascular treatment of unruptured cerebral aneurysms at university hospitals. Neurology 1999, 52, 1799. [Google Scholar] [CrossRef]
- Johnston, S.C.; Zhao, S.; Dudley, R.A.; Berman, M.F.; Gress, D.R. Treatment of unruptured cerebral aneurysms in California. Stroke 2001, 32, 597. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.C.; Wilson, C.B.; Halbach, V.V.; Higashida, R.T.; Dowd, C.F.; McDermott, M.W.; Applebury, C.B.; Farley, T.L.; Gress, D.R. Endovascular and surgical treatment of unruptured cerebral aneurysms: Comparison of risks. Ann. Neurol. 2000, 48, 11–19. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Janardhan, V.; Hanel, R.A.; Lanzino, G. Comparison of endovascular and surgical treatments for intracranial aneurysms: An evidence-based review. Lancet Neurol. 2007, 6, 816–825. [Google Scholar] [CrossRef]
- Molyneux, A.J.; Kerr, R.S.; Yu, L.M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar] [CrossRef]
- Sadato, A.; Hayakawa, M.; Adachi, K.; Nakahara, I.; Hirose, Y. Large residual volume, not low packing density, is the most influential risk factor for recanalization after coil embolization of cerebral aneurysms. PLoS ONE 2016, 11, e0155062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, A.S.; Molyneux, A.; Coon, A.L.; Saatci, I.; Szikora, I.; Baltacioglu, F.; Sultan, A.; Hoit, D.; Delgado Almandoz, J.E.; Elijovich, L.; et al. The safety and effectiveness of the Woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: Final 12-month results of the pivotal WEB Intrasaccular Therapy (WEB-IT) Study. J. NeuroInterv. Surg. 2019, 11, 924–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taschner, C.A.; Chapot, R.; Costalat, V.; Machi, P.; Courthéoux, P.; Barreau, X.; Berge, J.; Pierot, L.; Kadziolka, K.; Jean, B.; et al. Second-Generation hydrogel coils for the endovascular treatment of intracranial aneurysms. Stroke 2018, 49, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmi, G.; Vinuela, F.; Dion, J.; Duckwiler, G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J. Neurosurg. 1991, 75, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Vinuela, F.; Sepetka, I.; Macellari, V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J. Neurosurg. 1991, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G. Guglielmi detachable coils. J. NeuroInterv. Surg. 2018, 10, e5. [Google Scholar] [CrossRef]
- Tähtinen, O.I.; Vanninen, R.L.; Manninen, H.I.; Rautio, R.; Haapanen, A.; Niskakangas, T.; Rinne, J.; Keski-Nisula, L. Wide-necked intracranial aneurysms: Treatment with stent-assisted coil embolization during acute (<72 Hours) subarachnoid hemorrhage-experience in 61 consecutive patients. Radiology 2009, 253, 199–208. [Google Scholar] [CrossRef]
- Bendok, B.R.; Abi-Aad, K.R.; Ward, J.D.; Kniss, J.F.; Kwasny, M.J.; Rahme, R.J.; Aoun, S.G.; El Ahmadieh, T.Y.; El Tecle, N.E.; Zammar, S.G.; et al. The hydrogel endovascular aneurysm treatment trial (HEAT): A randomized controlled trial of the second-generation hydrogel coil. Neurosurgery 2020, 86, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Herting, S.M.; Ding, Y.; Boyle, A.J.; Dai, D.; Nash, L.D.; Asnafi, S.; Jakaitis, D.R.; Johnson, C.R.; Graul, L.M.; Yeh, C. In Vivo comparison of shape memory polymer foam-coated and bare metal coils for aneurysm occlusion in the rabbit elastase model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2466–2475. [Google Scholar] [CrossRef]
- Chueh, J.-Y.; Vedantham, S.; Wakhloo, A.K.; Carniato, S.L.; Puri, A.S.; Bzura, C.; Coffin, S.; Bogdanov, A.A.; Gounis, M.J. Aneurysm permeability following coil embolization: Packing density and coil distribution. J. NeuroInterv. Surg. 2015, 7, 676–681. [Google Scholar] [CrossRef] [Green Version]
- White, P.M.; Lewis, S.C.; Gholkar, A.; Sellar, R.J.; Nahser, H.; Cognard, C.; Forrester, L.; Wardlaw, J.M. Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): A randomised controlled trial. Lancet 2011, 377, 1655–1662. [Google Scholar] [CrossRef]
- Williams, A.; Millar, J.; Ditchfield, A.; Vundavalli, S.; Barker, S. Use of Hydrocoil in small aneurysms: Procedural safety, treatment efficacy and factors predicting complete occlusion. Interv. Neuroradiol. 2014, 20, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.-B.; Fan, Y.-M.; Zhang, J.-N. HydroSoft coil versus HydroCoil for endovascular aneurysm occlusion study: A single center experience. Eur. J. Radiol. 2011, 79, e42–e46. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Klink, R.; Chagnon, M.; Barnwell, S.L.; Evans, A.J.; Mocco, J.; Hoh, B.H.; Turk, A.S.; Turner, R.D.; Desal, H.; et al. Hydrogel versus bare platinum coils in patients with large or recurrent aneurysms prone to recurrence after endovascular treatment: A randomized controlled trial. Am. J. Neuroradiol. 2017, 38, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Xue, T.; Chen, Z.; Lin, W.; Xu, J.; Shen, X.; Wang, Z. Hydrogel coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms: A meta-analysis of randomized controlled trials. BMC Neurol. 2018, 18, 167. [Google Scholar] [CrossRef]
- Poupart, O.; Conti, R.; Schmocker, A.; Pancaldi, L.; Moser, C.; Nuss, K.M.; Sakar, M.S.; Dobrocky, T.; Grützmacher, H.; Mosimann, P.J.; et al. Pulsatile flow-induced fatigue-resistant photopolymerizable hydrogels for the treatment of intracranial aneurysms. Front. Bioeng. Biotechnol. 2021, 8, 619858. [Google Scholar] [CrossRef]
- Poupart, O.; Schmocker, A.; Conti, R.; Moser, C.; Nuss, K.M.; Grützmacher, H.; Mosimann, P.J.; Pioletti, D.P. In Vitro implementation of photopolymerizable hydrogels as a potential treatment of intracranial aneurysms. Front. Bioeng. Biotechnol. 2020, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Murayama, Y.; Viñuela, F.; Tateshima, S.; Viñuela, F.J.; Akiba, Y. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. Am. J. Neuroradiol. 2000, 21, 1726–1735. [Google Scholar]
- Tevah, J.; Senf, R.; Cruz, J.; Fava, M. Endovascular treatment of complex cerebral aneurysms with onyx hd-500® in 38 patients. J. Neuroradiol. 2011, 38, 283–290. [Google Scholar] [CrossRef]
- Chalouhi, N.; Tjoumakaris, S.; Gonzalez, L.F.; Hasan, D.; Alkhalili, K.; Dumont, A.S.; Rosenwasser, R.; Jabbour, P. Endovascular treatment of distal intracranial aneurysms with Onyx 18/34. Clin. Neurol. Neurosurg. 2013, 115, 2528–2532. [Google Scholar] [CrossRef]
- Klisch, J.; Sychra, V.; Strasilla, C.; Liebig, T.; Fiorella, D. The Woven Endobridge cerebral aneurysm embolization device (WEB II): Initial clinical experience. Neuroradiology 2011, 53, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Liebig, T.; Sychra, V.; Kadziolka, K.; Dorn, F.; Strasilla, C.; Kabbasch, C.; Klisch, J. Intrasaccular flow-disruption treatment of intracranial aneurysms: Preliminary results of a multicenter clinical study. Am. J. Neuroradiol. 2012, 33, 1232–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caroff, J.; Mihalea, C.; Klisch, J.; Strasilla, C.; Berlis, A.; Patankar, T.; Weber, W.; Behme, D.; Jacobsen, E.; Liebig, T.; et al. Single-Layer WEBs: Intrasaccular flow disrupters for aneurysm treatment—Feasibility results from a European study. Am. J. Neuroradiol. 2015, 36, 1942–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubicz, B.; Klisch, J.; Gauvrit, J.-Y.; Szikora, I.; Leonardi, M.; Liebig, T.; Nuzzi, N.; Boccardi, E.; Paola, F.; Holtmannspötter, M.; et al. WEB-DL Endovascular treatment of wide-neck bifurcation aneurysms: Short- and midterm results in a European Study. Am. J. Neuroradiol. 2014, 35, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caroff, J.; Mihalea, C.; Dargento, F.; Neki, H.; Ikka, L.; Benachour, N.; Moret, J.; Spelle, L. Woven Endobridge (WEB) Device for endovascular treatment of ruptured intracranial wide-neck aneurysms: A single-center experience. Neuroradiology 2014, 56, 755–761. [Google Scholar] [CrossRef]
- Clajus, C.; Strasilla, C.; Fiebig, T.; Sychra, V.; Fiorella, D.; Klisch, J. Initial and mid-term results from 108 consecutive patients with cerebral aneurysms treated with the WEB device. J. Neurointerv. Surg. 2017, 9, 411–417. [Google Scholar] [CrossRef]
- Maragkos, G.A.; Dmytriw, A.A.; Salem, M.M.; Tutino, V.M.; Meng, H.; Cognard, C.; Machi, P.; Krings, T.; Mendes Pereira, V. Overview of different flow diverters and flow dynamics. Neurosurgery 2020, 86, S21–S34. [Google Scholar] [CrossRef]
- Martínez-Galdámez, M.; Lamin, S.M.; Lagios, K.G.; Liebig, T.; Ciceri, E.F.; Chapot, R.; Stockx, L.; Chavda, S.; Kabbasch, C.; Faragò, G.; et al. Treatment of intracranial aneurysms using the pipeline flex embolization device with shield technology: Angiographic and safety outcomes at 1-year follow-up. J. NeuroInterv. Surg. 2019, 11, 396–399. [Google Scholar] [CrossRef]
- Trivelato, F.P.; Wajnberg, E.; Rezende, M.T.S.; Ulhôa, A.C.; Piske, R.L.; Abud, T.G.; de Castro-Afonso, L.H.; Abath, C.G.C.; Nakiri, G.S.; Araújo, J.F.S.; et al. Safety and effectiveness of the Pipeline Flex embolization device with shield technology for the treatment of intracranial aneurysms: Midterm results from a multicenter study. Neurosurgery 2020, 87, 104–111. [Google Scholar] [CrossRef]
- Li, L.; Gao, B.-L.; Wu, Q.-W.; Shao, Q.-J.; Wang, Z.-L.; Zhang, K.; Li, T.-X. Pipeline flex embolization device for the treatment of large unruptured posterior circulation aneurysms: Single-center experience. J. Clin. Neurosci. 2022, 96, 127–132. [Google Scholar] [CrossRef]
- Killer-Oberpfalzer, M.; Kocer, N.; Griessenauer, C.J.; Janssen, H.; Engelhorn, T.; Holtmannspötter, M.; Buhk, J.H.; Finkenzeller, T.; Fesl, G.; Trenkler, J.; et al. European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: The European flow-redirection intraluminal device study. Am. J. Neuroradiol. 2018, 39, 841–847. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhou, Y.; Li, Y.; Li, T.; Leng, B.; Zhang, P.; Liang, G.; Huang, Q.; Yang, P.F.; Shi, H.; et al. Parent artery reconstruction for large or giant cerebral aneurysms using the tubridge flow diverter: A Multicenter, Randomized, Controlled Clinical Trial (PARAT). Am. J. Neuroradiol. 2018, 39, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ren, Z.; He, S.; Zhu, Y.; Zhu, C. FTIR spectroscopic characterization of polyurethane-urea model hard segments (PUUMHS) based on three diamine chain extenders. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 66, 188–193. [Google Scholar] [CrossRef] [PubMed]
- De Beule, T.; Boulanger, T.; Heye, S.; van Rooij, W.; van Zwam, W.; Stockx, L. p64 flow diverter: Results in 108 patients from a single center. Interv. Neuroradiol. 2021, 27, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Rodriguez, J.N.; Small, W.; Eagleston, S.; Van de Water, J.; Maitland, D.J.; Wilson, T.S. Ultra low density and highly crosslinked biocompatible shape memory polyurethane foams. J. Polym. Sci. B Polym. Phys. 2012, 50, 724–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, J.; Hwang, W.; Jessen, S.L.; Keller, B.K.; Miller, M.W.; Tuzun, E.; Hartman, J.; Clubb, F.J.J.; Maitland, D.J. Comparison of shape memory polymer foam versus bare metal coil treatments in an in vivo porcine sidewall aneurysm model. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Jessen, S.L.; Friedemann, M.C.; Mullen, A.E.; Ginn-Hedman, A.-M.; Herting, S.M.; Maitland, D.J.; Clubb, F.J., Jr. Micro-CT and histopathology methods to assess host response of aneurysms treated with shape memory polymer foam-coated coils versus bare metal coil occlusion devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2238–2249. [Google Scholar] [CrossRef]
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Dev. 2010, 7, 357–379. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, I.A. Challenges of shape memory polymers: A review of the progress toward overcoming SMP’s limitations. Polym. Eng. Sci. 2008, 48, 2075–2089. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, N.; Zhuang, J.; Sun, Y.; Ren, F.; Zhang, W.; Hou, Z. Degradable poly(ether-ester-urethane)s based on well-defined aliphatic diurethane diisocyanate with excellent shape recovery properties at body temperature for biomedical application. Polymers 2019, 11, 1002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Xia, Y.; Wang, L.; Liu, L.; Liu, Y.; Leng, J. Conductive shape memory microfiber membranes with core–shell structures and electroactive performance. ACS Appl. Mater. Interfaces 2018, 10, 35526–35532. [Google Scholar] [CrossRef]
- Julich-Gruner, K.K.; Löwenberg, C.; Neffe, A.T.; Behl, M.; Lendlein, A. Recent trends in the chemistry of shape-memory polymers. Macromol. Chem. Phys. 2013, 214, 527–536. [Google Scholar] [CrossRef]
- Hu, J.; Chen, W.; Fan, P.; Gao, J.; Fang, G.; Cao, Z.; Peng, F. Epoxy shape memory polymer (SMP): Material preparation, uniaxial tensile tests and dynamic mechanical analysis. Polym. Test. 2017, 62, 335–341. [Google Scholar] [CrossRef]
- Kaloshkin, S.; Maksimkin, A.; Kaloshkina, M.; Zadorozhnyy, M.; Churyukanova, M. Shape memory behavior of ultra-high molecular weight polyethylene. MRS Online Proc. Libr. 2012, 1403, 207–213. [Google Scholar] [CrossRef]
- Kunkel, R.; Laurence, D.; Wang, J.; Robinson, D.; Scherrer, J.; Wu, Y.; Bohnstedt, B.; Chien, A.; Liu, Y.; Lee, C.-H. Synthesis and characterization of bio-compatible shape memory polymers with potential applications to endovascular embolization of intracranial aneurysms. J. Mech. Behav. Biomed. Mater. 2018, 88, 422–430. [Google Scholar] [CrossRef]
- Wang, J.; Kunkel, R.; Luo, J.; Li, Y.; Liu, H.; Bohnstedt, B.N.; Liu, Y.; Lee, C.-H. Shape memory polyurethane with porous architectures for potential applications in intracranial aneurysm treatment. Polymers 2019, 11, 631. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Luo, J.; Kunkel, R.; Saha, M.; Bohnstedt, B.N.; Lee, C.-H.; Liu, Y. Development of shape memory polymer nanocomposite foam for treatment of intracranial aneurysms. Mater. Lett. 2019, 250, 38–41. [Google Scholar] [CrossRef]
- Pineda-Castillo, S.A.; Luo, J.; Lee, H.; Bohnstedt, B.N.; Liu, Y.; Lee, C.-H. Effects of carbon nanotube infiltration on a shape memory polymer-based device for brain aneurysm therapeutics: Design and characterization of a Joule-heating triggering mechanism. Adv. Eng. Mater. 2021, 23, 2100322. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Foaming and Blowing Agents; ChemTec Publishing: Scarborough, UK, 2017. [Google Scholar]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Coll. Interfaces Sci. 2015, 222, 228–259. [Google Scholar] [CrossRef]
- Singhal, P.; Small, W., IV; Cosgriff-Hernandez, E.; Maitland, D.J.; Wilson, T.S. Low density biodegradable shape memory polyurethane foams for embolic biomedical applications. Acta Biomater. 2014, 10, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.M.; Fletcher, G.K.; Monroe, M.B.B.; Wierzbicki, M.A.; Nash, L.D.; Maitland, D.J. Shape memory polymer foams synthesized using glycerol and hexanetriol for enhanced degradation resistance. Polymers 2020, 12, 2290. [Google Scholar] [CrossRef] [PubMed]
- Petryk, N.M.; Haas, G.; Vakil, A.U.; Monroe, M.B.B. Shape memory polymer foams with tunable interconnectivity using off-the-shelf foaming components. J. Biomed. Mater. Res. Part A 2022, 110, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Srivastava, I.; Naguib, H.E. Development of multifunctional shape memory polymer foams. AIP Conf. Proc. 2015, 1664, 040005. [Google Scholar] [CrossRef]
- Vakil, A.U.; Petryk, N.M.; Shepherd, E.; Monroe, M.B.B. Biostable shape memory polymer foams for smart biomaterial applications. Polymers 2021, 13, 4084. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, T.; Liu, Y.; Leng, J. Microwave synthesis and actuation of shape memory polycaprolactone foams with high speed. Sci. Rep. 2015, 5, 11152. [Google Scholar] [CrossRef]
- De Nardo, L.; Bertoldi, S.; Tanzi, M.C.; Haugen, H.; Fare, S. Shape memory polymer cellular solid design for medical applications. Smart Mater. Struct. 2011, 20, 035004. [Google Scholar] [CrossRef]
- Zhang, D.; Petersen, K.M.; Grunlan, M.A. Inorganic–organic shape memory polymer (SMP) foams with highly tunable properties. ACS Appl. Mater. Interfaces 2013, 5, 186–191. [Google Scholar] [CrossRef]
- De Nardo, L.; Bertoldi, S.; Cigada, A.; Tanzi, M.C.; Haugen, H.J.; Farè, S. Preparation and characterization of shape memory polymer scaffolds via solvent casting/particulate leaching. J. Appl. Biomater. Funct. Mater. 2012, 10, 119–126. [Google Scholar] [CrossRef]
- Langford, T.; Mohammed, A.; Essa, K.; Elshaer, A.; Hassanin, H. 4D printing of origami structures for minimally invasive surgeries using functional scaffold. Appl. Sci. 2021, 11, 332. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Gisario, A.; Azizi, A.; Barletta, M. Investigation on shape recovery of 3D printed honeycomb sandwich structure. Polym. Adv. Technol. 2020, 31, 3361–3365. [Google Scholar] [CrossRef]
- Jia, H.; Gu, S.-Y.; Chang, K. 3D printed self-expandable vascular stents from biodegradable shape memory polymer. Adv. Polym. Technol. 2018, 37, 3222–3228. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, J. 4D-printing—fused deposition modeling printing and PolyJet printing with shape memory polymers composite. Fibers Polym. 2020, 21, 2364–2372. [Google Scholar] [CrossRef]
- Cersoli, T.; Cresanto, A.; Herberger, C.; MacDonald, E.; Cortes, P. 3D printed shape memory polymers produced via direct pellet extrusion. Micromachines 2021, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Garces, I.T.; Tang, T.; Ayranci, C. Cellulose nanocrystals reinforced shape memory polymer cardiovascular stent. Rapid Protot. J. 2020, 27, 37–44. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Guo, Y.; Zhang, H.; Zhang, Z. Multi-responsive shape memory polymer printed by fused deposition modeling technique. Exp. Polym. Lett. 2020, 14, 348–357. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Inverardi, N.; Briatico-Vangosa, F.; Baldi, F.; Pandini, S.; Scalet, G.; Auricchio, F.; Cerea, M.; Foppoli, A.; et al. Expandable drug delivery system for gastric retention based on shape memory polymers: Development via 4D printing and extrusion. Int. J. Pharm. 2019, 571, 118700. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, G.; Ehrmann, A. 3D printing of shape memory polymers. J. Appl. Polym. Sci. 2021, 138, 50847. [Google Scholar] [CrossRef]
- Han, X.-J.; Dong, Z.-Q.; Fan, M.-M.; Liu, Y.; Li, J.-H.; Wang, Y.-F.; Yuan, Q.-J.; Li, B.-J.; Zhang, S. pH-induced shape-memory polymers. Macromol. Rapid Commun. 2012, 33, 1055–1060. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W. Thermo/moisture responsive shape-memory polymer for possible surgery/operation inside living cells in future. Mater. Des. 2010, 31, 2684–2689. [Google Scholar] [CrossRef]
- Baer, G.M.; Small, W., IV; Wilson, T.S.; Benett, W.J.; Matthews, D.L.; Hartman, J.; Maitland, D.J. Fabrication and in vitro deployment of a laser-activated shape memory polymer vascular stent. Biomed. Eng. Online 2007, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Li, X.; Wang, Y.; Pan, Y.; Ding, X.; Peng, Y. A remote-activated shape memory polymer network employing vinyl-capped Fe3O4 nanoparticles as netpoints for durable performance. Smart Mater. Struct. 2014, 23, 085005. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; Yoo, H.J.; Cho, J.W. Influence of carbon nanotubes and polypyrrole on the thermal, mechanical and electroactive shape-memory properties of polyurethane nanocomposites. Compos. Sci. Technol. 2007, 67, 1920–1929. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Yan, B.; Liu, L.; Ren, J. Carbon nanotube–polyurethane shape memory nanocomposites with low trigger temperature. Eur. Polym. J. 2013, 49, 3867–3877. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Lan, X.; Leng, J.; Du, S. Review of electro-active shape-memory polymer composite. Compos. Sci. Technol. 2009, 69, 2064–2068. [Google Scholar] [CrossRef]
- Kim, I.; Cho, G. Polyurethane nanofiber strain sensors via in situ polymerization of polypyrrole and application to monitoring joint flexion. Smart Mater. Struct. 2018, 27, 075006. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; Goo, N.S.; Cho, J.W. Conducting shape memory polyurethane-Polypyrrole composites for an electroactive actuator. Macromol. Mater. Eng. 2005, 290, 1049–1055. [Google Scholar] [CrossRef]
- Leng, J.; Zhang, D.; Liu, Y.; Yu, K.; Lan, X. Study on the activation of styrene-based shape memory polymer by medium-infrared laser light. Appl. Phys. Lett. 2010, 96, 111905. [Google Scholar] [CrossRef]
- Schmidt, A.M. Electromagnetic activation of shape memory polymer networks containing magnetic nanoparticles. Macromol. Rapid Commun. 2006, 27, 1168–1172. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Y.; Leng, J. Shape memory polymer/CNT composites and their microwave induced shape memory behaviors. RSC Adv. 2014, 4, 2961–2968. [Google Scholar] [CrossRef]
- Meng, H.; Zheng, J.; Wen, X.; Cai, Z.; Zhang, J.; Chen, T. pH- and sugar-induced shape memory hydrogel based on reversible phenylboronic acid–diol ester bonds. Macromol. Rapid Commun. 2015, 36, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tian, T.; Wu, S.; Xiang, T.; Zhou, S. A pH-induced self-healable shape memory hydrogel with metal-coordination cross-links. Polym. Chem. 2019, 10, 1920–1929. [Google Scholar] [CrossRef]
- Huang, W.; Yang, B.; An, L.; Li, C.; Chan, Y. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005, 86, 114105. [Google Scholar] [CrossRef]
- Jung, Y.C.; So, H.H.; Cho, J.W. Water-responsive shape memory polyurethane block copolymer modified with polyhedral oligometric silsesquioxane. J. Macromol. Sci. Part B 2006, 45, 1189. [Google Scholar]
- Zhang, H.; Wang, H.; Zhong, W.; Du, Q. A novel type of shape memory polymer blend and the shape memory mechanism. Polymer 2009, 50, 1596–1601. [Google Scholar] [CrossRef]
- DiOrio, A.M.; Luo, X.; Lee, K.M.; Mather, P.T. A functionally graded shape memory polymer. Soft Matter 2011, 7, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Song, J. High performance shape memory polymer networks based on rigid nanoparticle cores. Proc.Natl. Acad. Sci. USA 2010, 107, 7652–7657. [Google Scholar] [CrossRef] [Green Version]
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.; Li, C.; Li, L.; Chor, J. Qualitative separation of the effects of carbon nano-powder and moisture on the glass transition temperature of polyurethane shape memory polymer. Scr. Mater. 2005, 53, 105–107. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.M.; Li, C.; Chor, J.H. Effects of moisture on the glass transition temperature of polyurethane shape memory polymer filled with nano-carbon powder. Eur. Polym. J. 2005, 41, 1123–1128. [Google Scholar] [CrossRef]
- Guo, B.; Chen, Y.; Lei, Y.; Zhang, L.; Zhou, W.Y.; Rabie, A.B.M.; Zhao, J. Biobased poly (propylene sebacate) as shape memory polymer with tunable switching temperature for potential biomedical applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, X.; Wang, Q.; Wang, T. A tough shape memory polymer with triple-shape memory and two-way shape memory properties. J. Mater. Chem. A 2014, 2, 4771–4778. [Google Scholar] [CrossRef]
- Min, C.; Cui, W.; Bei, J.; Wang, S. Biodegradable shape-memory polymer—Polylactide-co-poly (glycolide-co-caprolactone) multiblock copolymer. Polym. Adv. Technol. 2005, 16, 608–615. [Google Scholar] [CrossRef]
- Liu, G.; Ding, X.; Cao, Y.; Zheng, Z.; Peng, Y. Novel shape-memory polymer with two transition temperatures. Macromol. Rapid Commun. 2005, 26, 649–652. [Google Scholar] [CrossRef]
- Nji, J.; Li, G. A self-healing 3D woven fabric reinforced shape memory polymer composite for impact mitigation. Smart Mater. Struct. 2010, 19, 035007. [Google Scholar] [CrossRef]
- Nji, J.; Li, G. A biomimic shape memory polymer based self-healing particulate composite. Polymer 2010, 51, 6021–6029. [Google Scholar] [CrossRef]
- Boyle, A.J.; Wierzbicki, M.A.; Herting, S.; Weems, A.C.; Nathan, A.; Hwang, W.; Maitland, D.J. In Vitro performance of a shape memory polymer foam-coated coil embolization device. Med. Eng. Phys. 2017, 49, 56–62. [Google Scholar] [CrossRef]
- Small, W., IV; Buckley, P.R.; Wilson, T.S.; Benett, W.J.; Hartman, J.; Saloner, D.; Maitland, D.J. Shape memory polymer stent with expandable foam: A new concept for endovascular embolization of fusiform aneurysms. IEEE Trans. Biomed. Eng. 2007, 54, 1157–1160. [Google Scholar] [CrossRef]
- Imai, S.; Sakurai, K. An actuator of two-way behavior by using two kinds of shape memory polymers with different Tgs. Precis. Eng. 2013, 37, 572–579. [Google Scholar] [CrossRef]
- Wilson, T.S.; Bearinger, J.P.; Herberg, J.L.; Marion, J.E., III; Wright, W.J.; Evans, C.L.; Maitland, D.J. Shape memory polymers based on uniform aliphatic urethane networks. J. Appl. Polym. Sci. 2007, 106, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Zheng, N.; Hou, J.; Xu, Y.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Catalyst-free thermoset polyurethane with permanent shape reconfigurability and highly tunable triple-shape memory performance. ACS Macro Lett. 2017, 6, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, Q.; Li, L.; Chen, Q.; Niu, X.; Liu, J.; Pei, Q. Highly flexible silver nanowire electrodes for shape-memory polymer light-emitting diodes. Adv. Mater. 2011, 23, 664–668. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Wang, C.; Wang, T. High-strain shape memory polymer networks crosslinked by SiO2. J. Mater. Chem. 2011, 21, 9073–9078. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Wei, J.; Su, P.-C. 4D printing of high performance shape memory polymer using stereolithography. Mater. Des. 2017, 126, 219–225. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kida, S.; Nomura, M.; Hasegawa, M.; Yamashima, T.; Yamashita, J.; Matsui, O. Significance of volume embolization ratio as a predictor of recanalization on endovascular treatment of cerebral aneurysms with Guglielmi detachable coils. Interv. Neuroradiol. 2000, 6, 59–63. [Google Scholar] [CrossRef]
- Tamatani, S.; Ito, Y.; Abe, H.; Koike, T.; Takeuchi, S.; Tanaka, R. Evaluation of the stability of aneurysms after embolization using detachable coils: Correlation between stability of aneurysms and embolized volume of aneurysms. Am. J. Neuroradiol. 2002, 23, 762–767. [Google Scholar]
- Yang, B.; Huang, W.; Li, C.; Lee, C.; Li, L. On the effects of moisture in a polyurethane shape memory polymer. Smart Mater. Struct. 2003, 13, 191. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.; Li, C.; Li, L. Effects of moisture on the thermomechanical properties of a polyurethane shape memory polymer. Polymer 2006, 47, 1348–1356. [Google Scholar] [CrossRef]
- Tey, S.; Huang, W.; Sokolowski, W. Influence of long-term storage in cold hibernation on strain recovery and recovery stress of polyurethane shape memory polymer foam. Smart Mater. Struct. 2001, 10, 321. [Google Scholar] [CrossRef]
- De Nardo, L.; Alberti, R.; Cigada, A.; Yahia, L.; Tanzi, M.C.; Farè, S. Shape memory polymer foams for cerebral aneurysm reparation: Effects of plasma sterilization on physical properties and cytocompatibility. Acta Biomater. 2009, 5, 1508–1518. [Google Scholar] [CrossRef]
- De Nardo, L.; Moscatelli, M.; Silvi, F.; Tanzi, M.C.; Yahia, L.; Farè, S. Chemico-physical modifications induced by plasma and ozone sterilizations on shape memory polyurethane foams. J. Mater. Sci. Mater. Med. 2010, 21, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Strange, F.; Gruter, B.E.; Fandino, J.; Marbacher, S. Preclinical intracranial aneurysm aodels: A systematic review. Brain Sci. 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Device | Advantages | Limitations/Complications |
|---|---|---|
| GDCs (bare coils) | Gold standard for ICA endovascular treatment | Complete occlusion of 49–65%, high recurrence (44% in 5–6 years) and assisting devices required for the treatment of wide-necked ICAs |
| Hydrogel coils | Higher packing for improved aneurysm sac filling | Recurrence and complete occlusion are not better than GDCs |
| Liquid embolic devices | Potential higher packing and maximized aneurysm sac filling | High potential for device migration and FDA approval not granted yet |
| WEB | Designed for wide-necked aneurysms without assisting device | Limited occlusion, prone to compression in large ICAs, and very high recurrence (∼72%) |
| Flow diverter | Can treat complex ICA geometry and ideal for side-walled ICAs | Limited geometries can be treated, high delayed rupture and prone to immediate failure |
| TrelliX | Improved packing density | Not approved by the FDA yet (in clinical trial—NCT03988062) |
| SMP Material | Tested Activation Method | Features | Citation |
|---|---|---|---|
| Butadiene–styrene tri-block copolymer/PCL | Heat/water | Polymer blend with elastomeric and shape memory properties. | [96] |
| Norland Optical Adhesive 63 (propietary) | Heat | gradients have been reported. | [97] |
| Silsesquioxane nanoparticles/PLA | Heat | Long-term (>1 year) shape storage. | [98] |
| PCL-coated FeO /oligo(PCL) DMA/BA | Electromagnetism | Remote activation without reaching temperatures higher than . | [90] |
| Perfluorosulphonic acid ionomer | Heat | Multi-shape memory effect with distinctive thermal states. | [99] |
| Phenylboronic-acid-grafted alginate/PVA | pH | Stable shape storage at specific pH and sugar contents. | [92] |
| PU/carbon nanopowder | Joule heating | Carbon powder provides conductivity for electrical actuation. | [100,101] |
| Poly(propylene sebacate) | Heat | Ecologically sustainable material. | [102] |
| Polydopamine/PCL | Heat | Great thermal conductivity. | [103] |
| PLA/PPy | Joule heating | Polypyrrole provides conductivity for electrical triggering of shape recovery. PLA is biodegradable. | [51] |
| Olylactide-co-poly(glycolide-co-caprolactone) | Heat | Highly biodegradable. | [104] |
| Polymethyl metracrylate/PEG | Heat | Multi-shape memory effect with distinctive thermal states. | [105] |
| Polystyrene | Heat/IR light | IR activation provides a contactless actuation method (less invasive). Material can also be applied in catheter design. Self-healing properties. | [89,106] |
| Polystyrene/carbonanotubes | Electromagnetism | Microwave activation provides a minimally invasive actuation method. | [91] |
| Polystyrene/copolyester particulates | Heat | Self-healing properties. | [107] |
| PU | Moisture/pH/IR Light | Extensively applied for in-vitro and in-vivo occlusion of aneurysms. Furthermore, used in stent design. Facile control of and mechanical properties. Can be designed to be responsive to moisture, IR light, pH, and heat. Multi-shape memory effect with distinctive thermal states can also be achieved. | [55,81,94,95,108,109,110,111] |
| PU/CNT | Heat/Joule heating | CNTs provide conductivity for electrical triggering of shape recovery. | [58,85] |
| PU/PEG | Heat | Multi-shape memory effect. Facile tuning. | [112] |
| PU/PPy | Joule heating | Polypyrrole provides conductivity for electrical triggering of shape recovery. PLA is biodegradable. | [83,87,88] |
| Silver nanowire/PET | Heat | Reported for the design of light-emitting diodes. | [113] |
| SiO/PCL | Heat | SiO macroparticles provide improved mechanical properties (high strain). Biodegradable. | [114] |
| Tert-BA/di(ethylene glycol) diacrylate | Heat | It can be fabricated with stereolithography techniques. | [115] |
| -CDAlg/DETA-Alg | pH | Stable shape storage at specific pH. Biodegradable and biocompatible. | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Castillo, S.A.; Stiles, A.M.; Bohnstedt, B.N.; Lee, H.; Liu, Y.; Lee, C.-H. Shape Memory Polymer-Based Endovascular Devices: Design Criteria and Future Perspective. Polymers 2022, 14, 2526. https://doi.org/10.3390/polym14132526
Pineda-Castillo SA, Stiles AM, Bohnstedt BN, Lee H, Liu Y, Lee C-H. Shape Memory Polymer-Based Endovascular Devices: Design Criteria and Future Perspective. Polymers. 2022; 14(13):2526. https://doi.org/10.3390/polym14132526
Chicago/Turabian StylePineda-Castillo, Sergio A., Aryn M. Stiles, Bradley N. Bohnstedt, Hyowon Lee, Yingtao Liu, and Chung-Hao Lee. 2022. "Shape Memory Polymer-Based Endovascular Devices: Design Criteria and Future Perspective" Polymers 14, no. 13: 2526. https://doi.org/10.3390/polym14132526