Interfaces Based on Laser-Structured Arrays of Carbon Nanotubes with Albumin for Electrical Stimulation of Heart Cell Growth
Abstract
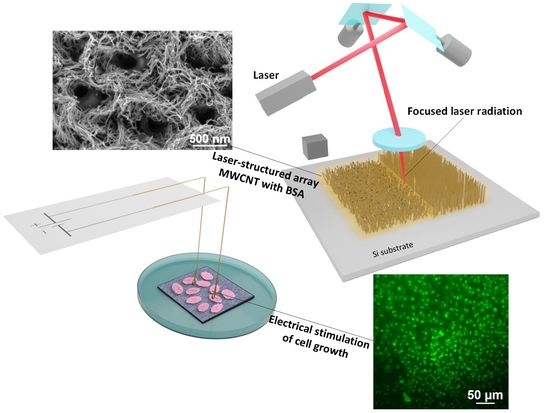
:1. Introduction
2. Materials and Methods
2.1. Formation of Samples from MWCNT Arrays with Albumin
2.2. Laser Structuring
2.3. Electron Microscopy
2.4. Raman Spectroscopy
2.5. Electrical Measurements
2.6. Fibroblast Cell Culture on the Samples
2.7. Cardiomyocytes Cell Culture on the Samples
2.8. Stimulation with Electric Field
2.9. Cell Viability Study
3. Results and Discussion
3.1. Morphology of Interfaces Based on MWCNT Arrays with BSA
3.2. Electrical Conductivity Measurements
3.3. Fibroblast Cell Growth on Interfaces
- -
- On laser-structured arrays, regardless of electrical stimulation, cells were grown more evenly and continuously distributed on the surface (i.e., homogeneous), whereas on samples without laser structuring, cells were distributed as separated “islands” (i.e., non-homogeneous).
- -
- Electrical stimulation of cells on the structured MWCNT arrays brought a significant effect on the cell growth density, exceeding the ones without stimulation almost three times.
3.4. Cardiomyocytes Cell Growth on CNT Arrays
- Cardiomyocytes growth rate was slower than in the case of fibroblasts; therefore, the initial cultivation period was increased to 4 days, and the time of electrical stimulation was reduced to 24 h, due to the high sensitivity of this type of cells to external influences.
- Laser structuring and electrical stimulation of cells together introduced a significant increase in the cell growth ratio similar to the previous experiment, which proved the efficacy of the combination of these methods for various biomedical applications.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chronopoulou, L.; Cacciotti, I.; Amalfitano, A.; Di Nitto, A.; D’Arienzo, V.; Nocca, G.; Palocci, C. Biosynthesis of innovative calcium phosphate/hydrogel composites: Physicochemical and biological characterisation. Nanotechnology 2021, 32, 095102. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-Based Coatings: Promising Strategies to Improve the Biocompatibility and Functionality of Materials Used in Biomedical Engineering. Adv. Mater. Interfaces 2020, 7, 2000850. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7. [Google Scholar] [CrossRef] [Green Version]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Yang, G.; Lv, F.; Wang, B.; Liu, L.; Yang, Q.; Wang, S. Multifunctional Non-Viral Delivery Systems Based on Conjugated Polymers. Macromol. Biosci. 2012, 12, 1600–1614. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Qian, C.G.; Zhu, S.; Feng, P.J.; Chen, Y.L.; Yu, J.C.; Tang, X.; Liu, Y.; Shen, Q.D. Conjugated Polymer Nanoparticles for Fluorescence Imaging and Sensing of Neurotransmitter Dopamine in Living Cells and the Brains of Zebrafish Larvae. ACS Appl. Mater. Interfaces 2015, 7, 18581–18589. [Google Scholar] [CrossRef]
- Li, K.; Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 6570–6597. [Google Scholar] [CrossRef] [Green Version]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bazan, G.C.; Liu, B. Conjugated-Polymer-Amplified Sensing, Imaging, and Therapy. Chem 2017, 2, 760–790. [Google Scholar] [CrossRef] [Green Version]
- Kotwal, A.; Schmidt, C.E. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials 2001, 22, 1055–1064. [Google Scholar] [CrossRef]
- Sensharma, P.; Madhumathi, G.; Jayant, R.D.; Jaiswal, A.K. Biomaterials and cells for neural tissue engineering: Current choices. Mater. Sci. Eng. C 2017, 77, 1302–1315. [Google Scholar] [CrossRef]
- Mihic, A.; Cui, Z.; Wu, J.; Vlacic, G.; Miyagi, Y.; Li, S.-H.; Lu, S.; Sung, H.-W.; Weisel, R.D.; Li, R.-K. A Conductive Polymer Hydrogel Supports Cell Electrical Signaling and Improves Cardiac Function After Implantation into Myocardial Infarct. Circulation 2015, 132, 772–784. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.G.; Li, H.; Chow, J.K.; Geissler, S.A.; Mcelroy, A.B.; Nguy, L.; Hernandez, D.S.; Schmidt, C.E. Conducting polymer-based multilayer films for instructive biomaterial coatings Dip coating direction Weakly aligned broblasts on multilayer lms: Glass-conducting polymer-chitosan-gelatin Passage of a DC current through the lm enhances cell alignment DC cu. Futur. Sci. OA 2015, 1, 79. [Google Scholar] [CrossRef] [Green Version]
- Mawad, D.; Mansfield, C.; Lauto, A.; Perbellini, F.; Nelson, G.W.; Tonkin, J.; Bello, S.O.; Carrad, D.J.; Micolich, A.P.; Mahat, M.M.; et al. A Conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2016, 2, e1601007. [Google Scholar] [CrossRef] [Green Version]
- Guex, A.G.; Puetzer, J.L.; Armgarth, A.; Littmann, E.; Stavrinidou, E.; Giannelis, E.P.; Malliaras, G.G.; Stevens, M.M. Highly porous scaffolds of PEDOT:PSS for bone tissue engineering. Acta Biomater. 2017, 62, 91–101. [Google Scholar] [CrossRef]
- Markov, A.; Maybeck, V.; Wolf, N.; Mayer, D.; Offenhäusser, A.; Wördenweber, R. Engineering of Neuron Growth and Enhancing Cell-Chip Communication via Mixed SAMs. ACS Appl. Mater. Interfaces 2018, 10, 18507–18514. [Google Scholar] [CrossRef]
- Cacciotti, I.; Valentini, M.; Raio, M.; Nanni, F. Design and development of advanced BaTiO3/MWCNTs/PVDF multi-layered systems for microwave applications. Compos. Struct. 2019, 224, 111075. [Google Scholar] [CrossRef]
- Huang, B. Carbon nanotubes and their polymeric composites: The applications in tissue engineering. Biomanufacturing Rev. 2020, 5, 3. [Google Scholar] [CrossRef]
- Zheng, T.; Pour Shahid Saeed Abadi, P.; Seo, J.; Cha, B.-H.; Miccoli, B.; Li, Y.-C.; Park, K.; Park, S.; Choi, S.-J.; Bayaniahangar, R.; et al. Biocompatible Carbon Nanotube-Based Hybrid Microfiber for Implantable Electrochemical Actuator and Flexible Electronic Applications. ACS Appl. Mater. Interfaces 2019, 11, 20615–20627. [Google Scholar] [CrossRef] [PubMed]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.; Verma, P.; Kumar, S. Electrical conductivity of CNT/polymer composites: 3D printing, measurements and modeling. Compos. Part B Eng. 2020, 183, 107600. [Google Scholar] [CrossRef]
- Teradal, N.L.; Jelinek, R. Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Kalmykov, A.; Johnson, N.; Cohen-Karni, T. Bioelectronics with nanocarbons. J. Mater. Chem. B 2018, 6, 7159–7178. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Wu, Z.; Wu, N.; Wang, Z.; Chen, X.; Wei, Y.; Zhang, P. Modulation of Osteogenesis in MC3T3-E1 Cells by Different Frequency Electrical Stimulation. PLoS ONE 2016, 11, e0154924. [Google Scholar] [CrossRef] [Green Version]
- O’Hearn, S.F.; Ackerman, B.J.; Mower, M.M. Paced monophasic and biphasic waveforms alter transmembrane potentials and metabolism of human fibroblasts. Biochem. Biophys. Rep. 2016, 8, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.; Iqbal, S.A.; Sebastian, A.; Colthurst, J.; Bayat, A. Degenerate Wave and Capacitive Coupling Increase Human MSC Invasion and Proliferation While Reducing Cytotoxicity in an In Vitro Wound Healing Model. PLoS ONE 2011, 6, e23404. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, X.; Zhao, R.; Wang, C.; Qiu, F.; Sun, B.; Ji, H.; Qiu, J.; Wang, C. Enhanced adhesion and proliferation of human umbilical vein endothelial cells on conductive PANI-PCL fiber scaffold by electrical stimulation. Mater. Sci. Eng. C 2017, 72, 106–112. [Google Scholar] [CrossRef]
- Tai, G.; Tai, M.; Zhao, M. Electrically stimulated cell migration and its contribution to wound healing. Burn. Trauma 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics 2016, 13, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chen, J.; Sun, H.; Qiu, X.; Mou, Y.; Liu, Z.; Zhao, Y.; Li, X.; Han, Y.; Duan, C.; et al. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep. 2015, 4, 3733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wei, L.; Lan, L.; Gao, Y.; Zhang, Q.; Dawit, H.; Mao, J.; Guo, L.; Shen, L.; Wang, L. Conductive biomaterials for cardiac repair: A review. Acta Biomater. 2022, 139, 157–178. [Google Scholar] [CrossRef]
- Hinderer, S.; Brauchle, E.; Schenke-Layland, K. Generation and Assessment of Functional Biomaterial Scaffolds for Applications in Cardiovascular Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2015, 4, 2326–2341. [Google Scholar] [CrossRef] [Green Version]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9, 448. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Osikoya, A.O.; Anku, W.W.; Shukla, S.K.; Govender, P.P. Hierarchically Assembled Two-dimensional Hybrid Nanointerfaces: A Platform for Bioelectronic Applications. Electroanalysis 2018, 30, 2339–2348. [Google Scholar] [CrossRef]
- Shin, S.R.; Shin, C.; Memic, A.; Shadmehr, S.; Miscuglio, M.; Jung, H.Y.; Jung, S.M.; Bae, H.; Khademhosseini, A.; Tang, X.S.; et al. Aligned Carbon Nanotube-Based Flexible Gel Substrates for Engineering Biohybrid Tissue Actuators. Adv. Funct. Mater. 2015, 25, 4486–4495. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Xu, Q.; Chen, X.; Li, W.; Guo, K.; Zhao, Y.; Wang, Q.; Zhang, Z.; Peng, H.; Li, Y.-G. Superaligned Carbon Nanotubes Guide Oriented Cell Growth and Promote Electrophysiological Homogeneity for Synthetic Cardiac Tissues. Adv. Mater. 2017, 29, 1702713. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Mizushima, D.; Ohtake, N.; Bauhofer, W.; Fiedler, B.; Schulte, K. On the manufacturing and electrical and mechanical properties of ultra-high wt.% fraction aligned MWCNT and randomly oriented CNT epoxy composites. Carbon 2015, 91, 275–290. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, J.; Li, Q.; Liu, L.; Liu, C.; Fan, S. Superaligned Carbon Nanotube Arrays, Films, and Yarns: A Road to Applications. Adv. Mater. 2011, 23, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, B.; Wu, Y.; Huang, Y.; Chen, Y. The production of horizontally aligned single-walled carbon nanotubes. Carbon 2011, 49, 4098–4110. [Google Scholar] [CrossRef]
- Sadeghpour, H.R.; Granger, B.E. Interaction of Laser Light and Electrons with Nanotubes. Phys. Scr. 2004, 110, 262. [Google Scholar] [CrossRef] [Green Version]
- Elmer, J.W.; Yaglioglu, O.; Schaeffer, R.D.; Kardos, G.; Derkach, O. Direct patterning of vertically aligned carbon nanotube arrays to 20μm pitch using focused laser beam micromachining. Carbon 2012, 50, 4114–4122. [Google Scholar] [CrossRef]
- Ushiba, S.; Shoji, S.; Masui, K.; Kono, J.; Kawata, S. Direct Laser Writing of 3D Architectures of Aligned Carbon Nanotubes. Adv. Mater. 2014, 26, 5653–5657. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, B. Optical orientation and shifting of a single multiwalled carbon nanotube. Light Sci. Appl. 2014, 3, e205. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hong, M.; Schmidt, M.; Zhong, M.; Malshe, A.; Huis in’tVeld, B.; Kovalenko, V. Laser nano-manufacturing—State of the art and challenges. CIRP Ann. 2011, 60, 735–755. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, J. Morphology adjustments of multi-walled carbon nanotubes by laser irradiation. Laser Phys. Lett. 2016, 13, 66001. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. Covalent Surface Functionalization of Bovine Serum Albumin to Magnesium Surface to Provide Robust Corrosion Inhibition and Enhance In Vitro Osteo-Inductivity. Polymers 2020, 12, 439. [Google Scholar] [CrossRef] [Green Version]
- Amdursky, N.; Wang, X.; Meredith, P.; Riley, D.J.; Payne, D.J.; Bradley, D.D.C.; Stevens, M.M. Electron Hopping Across Hemin-Doped Serum Albumin Mats on Centimeter-Length Scales. Adv. Mater. 2017, 29, 1700810. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, S.; Shapira, A.; Regev, O.; Nseir, N.; Zussman, E.; Dvir, T. Albumin fiber scaffolds for engineering functional cardiac tissues. Biotechnol. Bioeng. 2014, 111, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, B.; Latour, R.A. The Adherence of platelets to adsorbed albumin by receptor-mediated recognition of binding sites exposed by adsorption-induced unfolding. Biomaterials 2010, 31, 1036–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerasimenko, A.Y.; Ten, G.N.; Ryabkin, D.I.; Shcherbakova, N.E.; Morozova, E.A.; Ichkitidze, L.P. The study of the interaction mechanism between bovine serum albumin and single-walled carbon nanotubes depending on their diameter and concentration in solid nanocomposites by vibrational spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117682. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y. The interaction mechanism between bovine serum albumin and single-walled carbon nanotubes depending on their diameter and concentration in solid bionanocomposites. Proc. SPIE 2020, 113631, 113631P. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Kurilova, U.E.; Savelyev, M.S.; Murashko, D.T.; Glukhova, O.E. Laser fabrication of composite layers from biopolymers with branched 3D networks of single-walled carbon nanotubes for cardiovascular implants. Compos. Struct. 2021, 260, 113517. [Google Scholar] [CrossRef]
- Dassios, K.G.; Alafogianni, P.; Antiohos, S.K.; Leptokaridis, C.; Barkoula, N.-M.; Matikas, T.E. Optimization of Sonication Parameters for Homogeneous Surfactant-Assisted Dispersion of Multiwalled Carbon Nanotubes in Aqueous Solutions. J. Phys. Chem. C 2015, 119, 7506–7516. [Google Scholar] [CrossRef]
- Krause, B.; Mende, M.; Pötschke, P.; Petzold, G. Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon 2010, 48, 2746–2754. [Google Scholar] [CrossRef]
- Filho, A.G.S.; Jorio, A.; Samsonidze, G.G.; Dresselhaus, G.; Saito, R.; Dresselhaus, M.S. Raman spectroscopy for probing chemically/physically induced phenomena incarbon nanotubes. Nanotechnology 2003, 14, 1130. [Google Scholar] [CrossRef]
- Ramos, S.C.; Vasconcelos, G.; Antunes, E.F.; Lobo, A.O.; Trava-Airoldi, V.J.; Corat, E.J. Wettability control on vertically-aligned multi-walled carbon nanotube surfaces with oxygen pulsed DC plasma and CO2 laser treatments. Diam. Relat. Mater. 2010, 19, 752–755. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Alekseyev, A.V.; Lebedev, E.A.; Gavrilin, I.M.; Kitsuk, E.P.; Ryazanov, R.M.; Dudin, A.A.; Polokhin, A.A.; Gromov, D.G. Effect of the Plasma Functionalization of Carbon Nanotubes on the Formation of a Carbon Nanotube–Nickel Oxide Composite Electrode Material. Semiconductors 2018, 52, 1936–1941. [Google Scholar] [CrossRef]
- Poelma, R.H.; Sadeghian, H.; Koh, S.; Zhang, G.Q. Effects of single vacancy defect position on the stability of carbon nanotubes. Microelectron. Reliab. 2012, 52, 1279–1284. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Kuksin, A.V.; Shaman, Y.P.; Kitsyuk, E.P.; Fedorova, Y.O.; Sysa, A.V.; Pavlov, A.A.; Glukhova, O.E. Electrically Conductive Networks from Hybrids of Carbon Nanotubes and Graphene Created by Laser Radiation. Nanomaterials 2021, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Savelyev, M.S.; Gerasimenko, A.Y.; Vasilevsky, P.N.; Fedorova, Y.O.; Groth, T.; Ten, G.N.; Telyshev, D.V. Spectral analysis combined with nonlinear optical measurement of laser printed biopolymer composites comprising chitosan/SWCNT. Anal. Biochem. 2020, 598, 113710. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Kurilova, U.E.; Suetina, I.A.; Mezentseva, M.V.; Zubko, A.V.; Sekacheva, M.I.; Glukhova, O.E. Laser Technology for the Formation of Bioelectronic Nanocomposites Based on Single-Walled Carbon Nanotubes and Proteins with Different Structures, Electrical Conductivity and Biocompatibility. Appl. Sci. 2021, 11, 8036. [Google Scholar] [CrossRef]
- Demidenko, N.A.; Kuksin, A.V.; Molodykh, V.V.; Pyankov, E.S.; Ichkitidze, L.P.; Zaborova, V.A.; Tsymbal, A.A.; Tkachenko, S.A.; Shafaei, H.; Diachkova, E.; et al. Flexible Strain-Sensitive Silicone-CNT Sensor for Human Motion Detection. Bioengineering 2022, 9, 36. [Google Scholar] [CrossRef]
- Fasano, M.; Chiavazzo, E.; Asinari, P. Water transport control in carbon nanotube arrays. Nanoscale Res. Lett. 2014, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Rybolt, T.; Jordan, H. Interactions and Binding Energies in Carbon Nanotube Bundles. Appl. Nano 2021, 2, 128–147. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Kitsyuk, E.P.; Kuksin, A.V.; Ryazanov, R.M.; Savitskiy, A.I.; Savelyev, M.S.; Pavlov, A.A. Influence of laser structuring and barium nitrate treatment on morphology and electrophysical characteristics of vertically aligned carbon nanotube arrays. Diam. Relat. Mater. 2019, 96, 104–111. [Google Scholar] [CrossRef]
- Chatzichristos, A.; Hassan, J. Current Understanding of Water Properties inside Carbon Nanotubes. Nanomaterials 2022, 12, 174. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Glukhova, O.E.; Savostyanov, G.V.; Podgaetsky, V.M. Laser structuring of carbon nanotubes in the albumin matrix for the creation of composite biostructures. J. Biomed. Opt. 2017, 22, 065003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kichambare, P.D.; Chen, L.C.; Wang, C.T.; Ma, K.J.; Wu, C.T.; Chen, K.H. Laser irradiation of carbon nanotubes. Mater. Chem. Phys. 2001, 72, 218–222. [Google Scholar] [CrossRef]
- Chico, L.; Crespi, V.H.; Benedict, L.X.; Louie, S.G.; Cohen, M.L. Pure carbon nanoscale devices: Nanotube heterojunctions. Phys. Rev. Lett. 1996, 76, 971–974. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.; Sun, W.; Guo, Y.; Jia, H.; Yu, X.; Pan, G.; Wu, F. Molecular Targeting-Mediated Mild-Temperature Photothermal Therapy with a Smart Albumin-Based Nanodrug. Small 2019, 1900501. [Google Scholar] [CrossRef] [PubMed]
- Vivien, L.; Riehl, D.; Hache, F.; Anglaret, E. Nonlinear scattering origin in carbon nanotube suspensions. J. Nonlinear Opt. Phys. Mater. 2000, 09, 297–307. [Google Scholar] [CrossRef]
- Souza, N.; Zeiger, M.; Presser, V.; Mücklich, F. In situ tracking of defect healing and purification of single-wall carbon nanotubes with laser radiation by time-resolved Raman spectroscopy. RSC Adv. 2015, 5, 62149–62159. [Google Scholar] [CrossRef] [Green Version]
- Ulisse, G.; Brunetti, F.; Di Carlo, A.; Orlanducci, S.; Tamburri, E.; Guglielmotti, V.; Marrani, M.; Terranova, M.L. Carbon nanotubes field emission enhancement using a laser post treatment. J. Vac. Sci. Technol. B. Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2015, 33, 022203. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectroscopic investigation of carbon-based materials and their composites. Comparison between carbon nanotubes and carbon black. Chem. Phys. Lett. 2013, 590, 153–159. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Matsuki, N.; Takeda, M.; Ishikawa, T.; Kinjo, A.; Hayasaka, T.; Imai, Y.; Yamaguchi, T. Activation of caspases and apoptosis in response to low-voltage electric pulses. Oncol. Rep. 2010, 23, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Horn, R.; Patlak, J. Single channel currents from excised patches of muscle membrane. Proc. Natl. Acad. Sci. USA 1980, 77, 6930–6934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.-P.; Papasian, C.J.; Wang, J.Q.; Xiong, Z.-G. Modulation of acid-sensing ion channels: Molecular mechanisms and therapeutic potential. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 288–309. [Google Scholar]
- Santoni, G.; Morelli, M.B.; Amantini, C.; Santoni, M.; Nabissi, M.; Marinelli, O.; Santoni, A. “Immuno-Transient Receptor Potential Ion Channels”: The Role in Monocyte- and Macrophage-Mediated Inflammatory Responses. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling Cell Behavior Electrically: Current Views and Future Potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khitrin, A.K.; Khitrin, K.A.; Model, M.A. A model for membrane potential and intracellular ion distribution. Chem. Phys. Lipids 2014, 184, 76–81. [Google Scholar] [CrossRef]
- Ye, H.; Steiger, A. Neuron matters: Electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J. Neuroeng. Rehabil. 2015, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerencser, A.A.; Chinopoulos, C.; Birket, M.J.; Jastroch, M.; Vitelli, C.; Nicholls, D.G.; Brand, M.D. Quantitative measurement of mitochondrial membrane potential in cultured cells: Calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. 2012, 590, 2845–2871. [Google Scholar] [CrossRef] [Green Version]
- Markov, A.; Wördenweber, R.; Ichkitidze, L.; Gerasimenko, A.; Kurilova, U.; Suetina, I.; Mezentseva, M.; Offenhäusser, A.; Telyshev, D. Biocompatible SWCNT Conductive Composites for Biomedical Applications. Nanomaterials 2020, 10, 2492. [Google Scholar] [CrossRef]
- Ichkitidze, L.P.; Gerasimenko, A.Y.; Podgaetsky, V.M.; Selishchev, S.V. Layers with the tensoresistive properties and their possible applications in medicine. Mater. Phys. Mech. 2018, 37, 153–158. [Google Scholar] [CrossRef]
- Privalova, P.Y.; Gerasimenko, A.Y.; Petukhov, V.A.; Shimarov, E.N.; Suyetina, I.A.; Mezentseva, M.A. Electrical stimulation of cell growth on layers of composite material based on carbon nanotubes and polymers. AIP Conf. 2019, 2140, 020051. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Zhurbina, N.N.; Cherepanova, N.G.; Semak, A.E.; Zar, V.V.; Fedorova, Y.O.; Eganova, E.M.; Pavlov, A.A.; Telyshev, D.V.; Selishchev, S.V.; et al. Frame Coating of Single-Walled Carbon Nanotubes in Collagen on PET Fibers for Artificial Joint Ligaments. Int. J. Mol. Sci. 2020, 21, 6163. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, S.N.; Au, D.; Gale, B.K.; Brooks, B.D.; Brooks, A.E. Maximizing fibroblast adhesion on protein-coated surfaces using microfluidic cell printing. RSC Adv. 2015, 5, 104101–104109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ZHANG, X.; PRASAD, S.; Niyogi, S.; Morgan, A.; Ozkan, M.; Ozkan, C. Guided neurite growth on patterned carbon nanotubes. Sens. Actuators B Chem. 2005, 106, 843–850. [Google Scholar] [CrossRef]
- Memic, A.; Alhadrami, H.A.; Hussain, M.A.; Aldhahri, M.; Al Nowaiser, F.; Al-Hazmi, F.; Oklu, R.; Khademhosseini, A. Hydrogels 2.0: Improved properties with nanomaterial composites for biomedical applications. Biomed. Mater. 2015, 11, 014104. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Harun, W.S.W.; Yusoff, M.M. Highly porous of hydroxyethyl cellulose biocomposite scaffolds for tissue engineering. Int. J. Biol. Macromol. 2019, 122, 562–571. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, N.; Kim, Y.; Baik, M.S.; Yoo, M.; Kim, D.; Lee, W.J.; Kang, D.H.; Kim, S.; Lee, K.; et al. High-performance, polymer-based direct cellular interfaces for electrical stimulation and recording. NPG Asia Mater. 2018, 10, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Casanova, A.; Bettamin, L.; Blatche, M.C.; Mathieu, F.; Martin, H.; Gonzalez-Dunia, D.; Nicu, L.; Larrieu, G. Nanowire based bioprobes for electrical monitoring of electrogenic cells. J. Phys. Condens. Matter 2018, 30, 464001. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, Y.J.; Yoon, Y.J. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical Stimulation Promotes Wound Healing by Enhancing Dermal Fibroblast Activity and Promoting Myofibroblast Transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef] [Green Version]








| Stage | T, °C | Time, min | Pr, torr | P, W (RF/LF) | Gas Phase | ||||
|---|---|---|---|---|---|---|---|---|---|
| C2H2, (cm3/min) | Ar, (cm3/min) | NH3, (cm3/min) | H2, (cm3/min) | O2, (cm3/min) | |||||
| Oxidation | 280 | 10 | 3 | 100/0 | - | 100 | - | - | 100 |
| Reduction | 680 | 10 | 1.5 | 100/0 | - | 100 | 100 | 100 | - |
| Synthesis | 680 | 2 | 2 | 20/30 | 100 | 300 | 30 | 100 | - |
| Hydrophilic treatment | 280 | 0.3 | 1 | 0/20 | - | 100 | - | - | 100 |
| Element | Content in Initial Sample (%) (Figure 4a,b) | Content in Samples after Laser Treatment (%) | ||
|---|---|---|---|---|
| 0.011 J/cm2 (Figure 4c) | 0.013 J/cm2 (Figure 4c) | 0.015 J/cm2 (Figure 4e,f) | ||
| C | 70.7 ± 0.6 | 72.6 ± 0.8 | 75.4 ± 0.8 | 80.0 ± 0.7 |
| N | 16.3 ± 0.5 | 14.7 ± 0.6 | 12.6 ± 0.4 | 10.2 ± 0.5 |
| O | 8.6 ± 0.2 | 8.0 ± 0.3 | 7.7 ± 0.3 | 6.3 ± 0.2 |
| S | 2.6 ± 0.1 | 2.4 ± 0.1 | 1.8 ± 0.1 | 1.3 ± 0.3 |
| Na | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.2 |
| Initial Sample | Samples after Laser Exposure | ||||
|---|---|---|---|---|---|
| 0.011 J/cm2 | 0.013 J/cm2 | 0.015 J/cm2 | 0.017 J/cm2 | ||
| Specific conductivity value (S/m) | 100.7 ± 2 | 155.3 ± 4 | 174.8 ± 5 | 215.8 ± 10 | 112.2 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimenko, A.Y.; Kitsyuk, E.; Kurilova, U.E.; Suetina, I.A.; Russu, L.; Mezentseva, M.V.; Markov, A.; Narovlyansky, A.N.; Kravchenko, S.; Selishchev, S.V.; et al. Interfaces Based on Laser-Structured Arrays of Carbon Nanotubes with Albumin for Electrical Stimulation of Heart Cell Growth. Polymers 2022, 14, 1866. https://doi.org/10.3390/polym14091866
Gerasimenko AY, Kitsyuk E, Kurilova UE, Suetina IA, Russu L, Mezentseva MV, Markov A, Narovlyansky AN, Kravchenko S, Selishchev SV, et al. Interfaces Based on Laser-Structured Arrays of Carbon Nanotubes with Albumin for Electrical Stimulation of Heart Cell Growth. Polymers. 2022; 14(9):1866. https://doi.org/10.3390/polym14091866
Chicago/Turabian StyleGerasimenko, Alexander Yu., Evgeny Kitsyuk, Uliana E. Kurilova, Irina A. Suetina, Leonid Russu, Marina V. Mezentseva, Aleksandr Markov, Alexander N. Narovlyansky, Sergei Kravchenko, Sergey V. Selishchev, and et al. 2022. "Interfaces Based on Laser-Structured Arrays of Carbon Nanotubes with Albumin for Electrical Stimulation of Heart Cell Growth" Polymers 14, no. 9: 1866. https://doi.org/10.3390/polym14091866