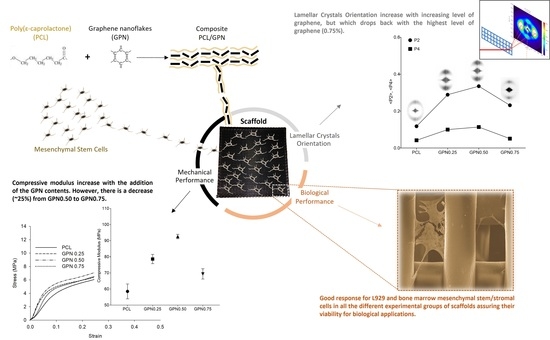
Additive Manufactured Poly(ε-caprolactone)-graphene Scaffolds: Lamellar Crystal Orientation, Mechanical Properties and Biological Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCL/GPN Composite
2.3. Scaffold Design and Fabrication
2.4. Characterisation of PCL/GPN Scaffolds
2.4.1. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.4.2. Measurement of the Electrical Conductivity of the PCL/GPN Composites
2.4.3. Surface Hydrophilicity Characterization
2.4.4. Mechanical Testing of the 3D PCL/GPN Scaffolds
2.4.5. SAXS Experiments
2.5. PCL/GPN Scaffold Biological Performance
2.5.1. In Vitro Cytotoxicity Evaluation
2.5.2. Cell Seeding and Culture on 3D PCL/GPN Scaffolds
2.5.3. Cell Proliferation (Alamar Blue) Assay
2.5.4. BM MSC Viability and Morphological Analysis
2.6. Statistical Analysis
3. Results
3.1. Processing Parameter Impact on Material Properties
3.2. Impact of GPN on Scaffold Properties
3.3. Impact of GPN on Cell–Scaffold Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamburaci, S.; Tihminlioglu, F. Biosilica incorporated 3D porous scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2018, 91, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Kamali, A.; Fard, M.R.B. Microstructure and characteristic properties of gelatin/chitosan scaffold prepared by the freeze-gelation method. Mater. Res. Express 2019, 6, 115404. [Google Scholar] [CrossRef]
- Dalgıç, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom shell incorporated PHBV/PCL-pullulan co-electrospun scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- Huh, J.T.; Yoo, J.J.; Atala, A.; Lee, S.J. Three-Dimensional Bioprinting for Tissue Engineering; INC: New York, NY, USA, 2020; ISBN 9780128184226. [Google Scholar]
- Kengla, C.; Lee, S.J.; Yoo, J.J.; Atala, A. 3-D Bioprinting Technologies for Tissue Engineering Applications, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081026632. [Google Scholar]
- Amado, S.; Morouço, P.; Pascoal-Faria, P.; Alves, N. Tailoring Bioengineered Scaffolds for Regenerative Medicine. In Biomaterials in Regenerative Medicine Research; Dobrzański, L.A., Ed.; IntechOpen: London, UK, 2018; ISBN 978-953-51-3777-1. [Google Scholar]
- Morouço, P.; Biscaia, S.; Viana, T.; Franco, M.; Malça, C.; Mateus, A.; Moura, C.; Ferreira, F.C.; Mitchell, G.; Alves, N. Fabrication of Poly(ε-caprolactone) Scaffolds Reinforced with Cellulose Nanofibers, with and without the Addition of Hydroxyapatite Nanoparticles. BioMed Res. Int. 2016, 2016, 1596157. [Google Scholar] [CrossRef] [Green Version]
- Nasr, S.M.; Rabiee, N.; Hajebi, S.; Ahmadi, S.; Fatahi, Y.; Hosseini, M.; Bagherzadeh, M.; Ghadiri, A.M.; Rabiee, M.; Jajarmi, V.; et al. Biodegradable Nanopolymers in Cardiac Tissue Engineering: From Concept Towards Nanomedicine. Int. J. Nanomed. 2020, 15, 4205–4224. [Google Scholar] [CrossRef]
- Idumah, C.I. Progress in polymer nanocomposites for bone regeneration and engineering. Polym. Polym. Compos. 2020, 29, 509–527. [Google Scholar] [CrossRef]
- Kuila, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- The Graphene Council “GRAPHENE CLASSIFICATION FRAMEWORK”. Available online: https://www.thegraphenecouncil.org/ (accessed on 5 January 2022).
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Atmaca, H. Prechondrogenic ATDC5 cell response to graphene/multi-walled carbon nanotube-containing porous polycaprolactone biocomposite scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 1154–1166. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D graphene-containing structures for tissue engineering. Mater. Today Chem. 2019, 14, 100199. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Yousefi, N.; Sun, X.; Lin, X.; Shen, X.; Jia, J.; Zhang, B.; Tang, B.; Chan, M.; Kim, J.-K. Highly Aligned Graphene/Polymer Nanocomposites with Excellent Dielectric Properties for High-Performance Electromagnetic Interference Shielding. Adv. Mater. 2014, 26, 5480–5487. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K.; et al. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111525. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Mansour, S.F.; Al-Wafi, R.; Afifi, M.; Uskoković, V. Gold as a dopant in selenium-containing carbonated hydroxyapatite fillers of nanofibrous ε-polycaprolactone scaffolds for tissue engineering. Int. J. Pharm. 2020, 577, 118950. [Google Scholar] [CrossRef]
- Samani, D.A.; Doostmohammadi, A.; Nilforoushan, M.R.; Nazari, H. Electrospun Polycaprolactone/Graphene/Baghdadite Composite Nanofibres with Improved Mechanical and Biological Properties. Fibers Polym. 2019, 20, 982–990. [Google Scholar] [CrossRef]
- Ginestra, P. Manufacturing of polycaprolactone—Graphene fibers for nerve tissue engineering. J. Mech. Behav. Biomed. Mater. 2019, 100, 103387. [Google Scholar] [CrossRef] [Green Version]
- Evlashin, S.; Dyakonov, P.; Tarkhov, M.; Dagesyan, S.; Rodionov, S.; Shpichka, A.; Kostenko, M.; Konev, S.; Sergeichev, I.; Timashev, P.; et al. Flexible Polycaprolactone and Polycaprolactone/Graphene Sca ff olds for Tissue Engineering. Materials 2019, 12, 2991. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bartolo, P. Engineered 3D printed poly(ε-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Sayyar, S.; Molino, P.J.; Wallace, G.G. A robust 3D printed multilayer conductive graphene/polycaprolactone composite electrode. Mater. Chem. Front. 2020, 4, 1664–1670. [Google Scholar] [CrossRef]
- Mohan, S.; Olley, R.H.; Vaughan, A.S.; Mitchell, G.R. Evaluating Scales of Structures. In Controlling the Morphology of Polymers; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319393223. [Google Scholar]
- Chu, B.; Hsiao, B.S. Small-Angle X-ray Scattering of Polymers. Chem. Rev. 2001, 101, 1727–1761. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.A.; Bartolo, P.J.; Mota, C.M.; Mateus, A.J. Processo e equipamento de fabrico rápido por bioextrusão. Port. Pat. Appl. 2010, 104247, 210. [Google Scholar]
- Mateus, A.J.; Almeida, H.A.; Ferreira, N.M.; Alves, N.M.; Bártolo, P.J.; Mota, C.M.; de Sousa, J.P. Bioextrusion for tissue engineering applications. In Virtual Rapid Manuf. Adv. Res. Virtual Rapid Prototyp.; Taylor and Francis: London, UK, 2007. [Google Scholar]
- Pattanashetti, N.A.; Biscaia, S.; Moura, C.; Mitchell, G.R.; Kariduraganavar, M.Y. Development of novel 3D scaffolds using BioExtruder by the incorporation of silica into polycaprolactone matrix for bone tissue engineering. Mater. Today Commun. 2019, 21, 100651. [Google Scholar] [CrossRef]
- Ângelo, D.F.; Wang, Y.; Morouco, P.; Monje, F.; Mónico, L.; González-Garcia, R.; Moura, C.; Alves, N.; Sanz, D.; Gao, J.; et al. A randomized controlled preclinical trial on 3 interposal temporomandibular joint disc implants: TEMPOJIMS—Phase 2. J. Tissue Eng. Regen. Med. 2021, 15, 852–868. [Google Scholar] [CrossRef]
- Wurm, A.; Lellinger, D.; Minakov, A.A.; Skipa, T.; Pötschke, P.; Nicula, R.; Alig, I.; Schick, C. Crystallization of poly(ε-caprolactone)/MWCNT composites: A combined SAXS/WAXS, electrical and thermal conductivity study. Polymer 2014, 55, 2220–2232. [Google Scholar] [CrossRef]
- Xin, H.M.; Jun, J. Poly(ε-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 2014, 21, 2727–2741. [Google Scholar] [CrossRef]
- Sarasini, F.; Tirillò, J.; Puglia, D.; Dominici, F.; Santulli, C.; Boimau, K.; Valente, T.; Torre, L. Biodegradable polycaprolactone-based composites reinforced with ramie and borassus fibres. Compos. Struct. 2017, 167, 20–29. [Google Scholar] [CrossRef]
- Garrudo, F.F.F.; Udangawa, R.N.; Hoffman, P.R.; Sordini, L.; Chapman, C.A.; Mikael, P.E.; Ferreira, F.A.; Silva, J.C.; Rodrigues, C.A.V.; Cabral, J.M.S.; et al. Polybenzimidazole nanofibers for neural stem cell culture. Mater. Today Chem. 2019, 14, 100185. [Google Scholar] [CrossRef]
- Garrudo, F.F.; Mikael, P.E.; Rodrigues, C.A.; Udangawa, R.W.; Paradiso, P.; Chapman, C.A.; Hoffman, P.; Colaço, R.; Cabral, J.M.; Morgado, J.; et al. Polyaniline-polycaprolactone fibers for neural applications: Electroconductivity enhanced by pseudo-doping. Mater. Sci. Eng. C 2021, 120, 111680. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization [ISO]: London, UK, 2009.
- Wegmeyer, H.; Bröske, A.-M.; Leddin, M.; Kuentzer, K.; Nisslbeck, A.K.; Hupfeld, J.; Wiechmann, K.; Kuhlen, J.; Von Schwerin, C.; Stein, C.; et al. Mesenchymal Stromal Cell Characteristics Vary Depending on Their Origin. Stem Cells Dev. 2013, 22, 2606–2618. [Google Scholar] [CrossRef] [Green Version]
- Laroye, C.; Gauthier, M.; Antonot, H.; Decot, V.; Reppel, L.; Bensoussan, D. Mesenchymal Stem/Stromal Cell Production Compliant with Good Manufacturing Practice: Comparison between Bone Marrow, the Gold Standard Adult Source, and Wharton’s Jelly, an Extraembryonic Source. J. Clin. Med. 2019, 8, 2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Heino, T.; Hentunen, T.A. Differentiation of Osteoblasts and Osteocytes from Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2008, 3, 131–145. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, F.; Andrade, P.Z.; Boura, J.S.; Abecasis, M.M.; da Silva, C.L.; Cabral, J.M. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 2010, 223, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Moura, C.S.; Borrecho, G.; de Matos, A.P.A.; Cabral, J.M.S.; Linhardt, R.J.; Ferreira, F.C. Effects of glycosaminoglycan supplementation in the chondrogenic differentiation of bone marrow- and synovial- derived mesenchymal stem/stromal cells on 3D-extruded poly (ε-caprolactone) scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 207–222. [Google Scholar] [CrossRef]
- Mitchell, G.R.; Saengsuwan, S.; Bualek-Limcharoen, S. Evaluation of preferred orientation in multi-component polymer systems using X-ray scattering procedures. Prog. Colloid Polym. Sci. 2005, 130, 149–158. [Google Scholar] [CrossRef]
- Strobl, G.R.; Schneider, M. Direct evaluation of the electron density correlation function of partially crystalline polymers. J. Polym. Sci. 1980, 18, 1343–1359. [Google Scholar] [CrossRef]
- Blázquez-Blázquez, E.; Pérez, E.; Lorenzo, V.; Cerrada, M.L. Crystalline Characteristics and Their Influence in the Mechanical Performance in Poly(ε-Caprolactone)/High Density Polyethylene Blends. Polymers 2019, 11, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tojeira, A.; Biscaia, S.S.; Viana, T.Q.; Sousa, I.S.; Mitchell, G.R. Controlling Morphology in 3D Printing. In Controlling the Morphology of Polymers; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Zhang, J.; Qiu, Z. Morphology, Crystallization Behavior, and Dynamic Mechanical Properties of Biodegradable Poly(ε-caprolactone)/Thermally Reduced Graphene Nanocomposites. Ind. Eng. Chem. Res. 2011, 50, 13885–13891. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon N. Y. 2013, 52, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T.; et al. 3D Printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.-X.; Hou, Y.; Bartolo, P.; Chiang, W.-H. Investigations of Graphene and Nitrogen-Doped Graphene Enhanced Polycaprolactone 3D Scaffolds for Bone Tissue Engineering. Nanomaterials 2021, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.Y.; Sayyar, S.; Wallace, G.G. Effect of Graphene Addition on Polycaprolactone Scaffolds Fabricated Using Melt-Electrowriting. Polymers 2022, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, M.; Chen, Y.; Gao, J. Thermal stability of graphene in inert atmosphere at high temperature. J. Solid State Chem. 2019, 276, 100–103. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Vitus, V.; Ibrahim, F.; Safwani, W.; Kamarul, W. Modelling of Stem Cells Microenvironment Using Carbon-Based Scaffold for Tissue Engineering Application—A Review. Polymers 2021, 13, 4058. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Fan, F.-Y.; Shen, Y.-K.; Wang, C.-H.; Huang, Y.-T.; Chern, M.-J.; Wang, Y.-H.; Wang, L. 3D poly-ε-caprolactone/graphene porous scaffolds for bone tissue engineering. Colloids Surfaces A 2020, 606, 125393. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Kotrotsos, A.; Fouriki, K. Graphene Nanoplatelet- and Hydroxyapatite-Doped Supramolecular Electrospun Fibers as Potential Materials for Tissue Engineering and Cell Culture. Int. J. Mol. Sci. 2019, 20, 1674. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Mayer, A.; Alvarado-Tenorio, B.; Romo-Uribe, A.; Benavente, R.; Jaffe, M.; Molina-Ocampo, A. Nanostructure reorganization in a thermotropic copolyester. A simultaneous WAXS and SAXS study. Polym. Adv. Technol. 2016, 27, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Nogales, A.; Gutiérrez-Fernández, E.; García-Gutiérrez, M.-C.; Ezquerra, T.A.; Rebollar, E.; Šics, I.; Malfois, M.; Gaidukovs, S.; Gecis, E.; Celms, K.; et al. Structure Development in Polymers during Fused Filament Fabrication (FFF): An in Situ Small- and Wide-Angle X-ray Scattering Study Using Synchrotron Radiation. Macromolecules 2019, 52, 9715–9723. [Google Scholar] [CrossRef]
- Tojeira, A.; Biscaia, S.S.; Viana, T.Q.; Sousa, I.S.; Mitchell, G.R. Controlling the Morphology of Polymers. In Controlling the Morphology of Polymers; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kyriakidou, K.; Lucarini, G.; Zizzi, A.; Salvolini, E.; Belmonte, M.M.; Mollica, F.; Gloria, A.; Ambrosio, L. Dynamic Co-Seeding of Osteoblast and Endothelial Cells on 3D Polycaprolactone Scaffolds for Enhanced Bone Tissue Engineering. J. Bioact. Compat. Polym. 2008, 23, 227–243. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bartolo, P.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef]
- Ciurana, J.; Serenóa, L.; Vallès, È. Selecting Process Parameters in RepRap Additive Manufacturing System for PLA Scaffolds Manufacture. Procedia CIRP 2013, 5, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.-Y.; Kim, H.-E.; Koh, Y.-H. Production, mechanical properties and in vitro biocompatibility of highly aligned porous poly(ε-caprolactone) (PCL)/hydroxyapatite (HA) scaffolds. J. Porous Mater. 2013, 20, 701–708. [Google Scholar] [CrossRef]
- Sridhar, V.; Lee, I.; Chun, H.H.; Park, H. Graphene reinforced biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) nano-composites. Express Polym. Lett. 2013, 7, 320–328. [Google Scholar] [CrossRef]
- Kai, W.; Hirota, Y.; Hua, L.; Inoue, Y. Thermal and Mechanical Properties of a Poly (ε-caprolactone)/Graphite Oxide Composite. J. Appl. Polym. Sci. 2007, 107, 1395–1400. [Google Scholar] [CrossRef]
- Xie, W.; Song, F.; Wang, R.; Sun, S.; Li, M.; Fan, Z.; Liu, B.; Zhang, Q.; Wang, J. Mechanically Robust 3D Graphene–Hydroxyapatite Hybrid Bioscaffolds with Enhanced Osteoconductive and Biocompatible Performance. Crystals 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Tjong, S.C. Low percolation threshold of graphene/polymer composites prepared by solvothermal reduction of graphene oxide in the polymer solution. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gkourmpis, T.; Gaska, K.; Tranchida, D.; Gitsas, A.; Müller, C.; Matic, A.; Kádár, R. Melt-Mixed 3D Hierarchical Graphene/Polypropylene Nanocomposites with Low Electrical Percolation Threshold. Nanomaterials 2019, 9, 1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.S.; Silva, J.C.; Faria, S.; Fernandes, P.R.; da Silva, C.L.; Cabral, J.M.S.; Linhardt, R.; Bártolo, P.J.; Ferreira, F.C. Chondrogenic differentiation of mesenchymal stem/stromal cells on 3D porous poly (ε-caprolactone) scaffolds: Effects of material alkaline treatment and chondroitin sulfate supplementation. J. Biosci. Bioeng. 2020, 129, 756–764. [Google Scholar] [CrossRef]
- Hong, G.; Han, Y.; Schutzius, T.; Wang, Y.; Pan, Y.; Hu, M.; Jie, J.; Sharma, C.S.; Müller, U.; Poulikakos, D. On the Mechanism of Hydrophilicity of Graphene. Nano Lett. 2016, 16, 4447–4453. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Atmaca, H. Effect of pore architecture on the mesenchymal stem cell responses to graphene/polycaprolactone scaffolds prepared by solvent casting and robocasting. J. Porous Mater. 2020, 27, 49–61. [Google Scholar] [CrossRef]
- Basal, O.; Ozmen, O.; Deliormanli, A.M. Effect of Polycaprolactone Scaffolds Containing Different Weights of Graphene on Healing in Large Osteochondral Defect Model. J. Biomater. Sci. Polym. Ed. 2022, 21, 1–17. [Google Scholar] [CrossRef]








| Design parameters | Filament gap | FG | [µm] | 350 |
| Filament distance | FD | [µm] | 650 | |
| Filament width | FW | [µm] | 300 | |
| Slice thickness | ST | [µm] | 280 | |
| Processing parameters | Melting temperature | MT | [°C] | 80 |
| Deposition velocity | DV | [mm/min] | 480 | |
| Screw rotation velocity | SRV | [rpm] | 30 | |
| Nozzle diameter | ND | [µm] | 300 |
| Pre-Processing | Post-Processing | |||||||
|---|---|---|---|---|---|---|---|---|
| PCL | GPN0.25 | GPN0.50 | GPN0.75 | PCL | GPN0.25 | GPN0.50 | GPN0.75 | |
| Tc [°C] | 35.84 ± 1.65 | *** 39.67 ± 0.18 | *** 39.68 ± 0.01 | *** 39.84 ± 0.18 | 38.20 ± 0.11 | *** 40.68 ± 0.01 | *** 41.59 ± 0.12 | *** 42.54 ± 0.06 |
| Tm [°C] | 58.84 ± 0.27 | 59.06 ± 0.05 | 59.24 ± 0.06 | 59.26 ± 0.05 | 59.06 ± 0.04 | 59.29 ± 0.28 | 59.30 ± 0.01 | 59.45 ± 0.08 |
| ΔHm [J/g] | 58.26 ± 031 | 58.40 ± 1.18 | ** 59.60 ± 1.60 | 58.41 ± 0.33 | 58.08 ± 0.98 | *** 61.00 ± 1.45 | *** 62.96 ± 0.63 | *** 62.95 ± 0.75 |
| Xc | 0.42 ± 0.00 | 0.42 ± 0.01 | 0.43 ± 0.01 | 0.42 ± 0.00 | 0.42 ± 0.01 | 0.44 ± 0.01 | 0.45 ± 0.01 | 0.45 ± 0.01 |
| ΔM [%] | 99.14 ± 0.30 | 99.14 ± 0.85 | 99.39 ± 0.54 | 99.50 ± 0.32 | 99.58 ± 0.44 | 98.84 ± 0.59 | 99.43 ± 0.56 | *** 98.06 ± 0.88 |
| P DTG [°C] | 410.22 ± 0.15 | *** 406.73 ± 0.25 | 410.23 ± 0.12 | 409.79 ± 0.42 | 411.14 ± 0.52 | 411.24 ± 0.49 | 411.19 ± 0.58 | 411.43 ± 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscaia, S.; Silva, J.C.; Moura, C.; Viana, T.; Tojeira, A.; Mitchell, G.R.; Pascoal-Faria, P.; Ferreira, F.C.; Alves, N. Additive Manufactured Poly(ε-caprolactone)-graphene Scaffolds: Lamellar Crystal Orientation, Mechanical Properties and Biological Performance. Polymers 2022, 14, 1669. https://doi.org/10.3390/polym14091669
Biscaia S, Silva JC, Moura C, Viana T, Tojeira A, Mitchell GR, Pascoal-Faria P, Ferreira FC, Alves N. Additive Manufactured Poly(ε-caprolactone)-graphene Scaffolds: Lamellar Crystal Orientation, Mechanical Properties and Biological Performance. Polymers. 2022; 14(9):1669. https://doi.org/10.3390/polym14091669
Chicago/Turabian StyleBiscaia, Sara, João C. Silva, Carla Moura, Tânia Viana, Ana Tojeira, Geoffrey R. Mitchell, Paula Pascoal-Faria, Frederico Castelo Ferreira, and Nuno Alves. 2022. "Additive Manufactured Poly(ε-caprolactone)-graphene Scaffolds: Lamellar Crystal Orientation, Mechanical Properties and Biological Performance" Polymers 14, no. 9: 1669. https://doi.org/10.3390/polym14091669