Surface Relief Grating on Chitosan-N,N-dimethyl-4-(2-pyridylazo)aniline Thin Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chitosan–PADA Solution Preparation
2.2. Chitosan–PADA Film Deposition
2.3. Characterization of the Chi–PADA Films
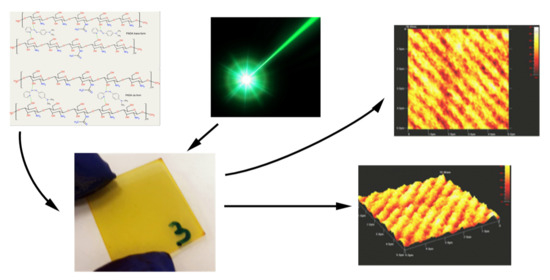
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merino, E.; Ribagorda, M. Control over molecular motion using the cis–trans photoisomerization of the azo group. Beilstein J. Org. Chem. 2012, 8, 1071–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.M.; Li, X.; Islam, M.R.; Wei, M.; Serpe, M.J. Light switchable optical materials from azobenzene crosslinked poly(N-isopropylacrylamide)-based microgels. J Mater. Chem. C 2014, 2, 6961–6965. [Google Scholar] [CrossRef] [Green Version]
- Schab-Balcerzak, E.; Sobolewska, A.; Stumpe, J.; Hamryszak, L.; Bujak, P. Surface relief gratings in azobenzene supramolecular systems based on polyimides. Optical Mat. 2012, 35, 155–167. [Google Scholar] [CrossRef]
- Yager, K.G.; Barrett, C.J. Novel photo-switching using azobenzene functional materials. J. Photochem. Photobiol. A Chem. 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Sekkat, Z.; Knoll, W. Photoreactive Organic Thin Films; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Kumar, J.; Li, L.; Jiang, X.L.; Kim, D.-Y.; Lee, T.S.; Tripathy, S. Gradient force: The mechanism for surface relief grating formation in azobenzene functionalized polymers. Appl. Phys. Lett. 1998, 72, 2096–2098. [Google Scholar] [CrossRef]
- Pawlicka, A.; Sabadini, R.C.; Nunzi, J.M. Reversible light-induced solubility of disperse red 1 dye in a hydroxypropyl cellulose matrix. Cellulose 2018, 25, 2083–2090. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Forni, A.; Gorynsztejn–Leben, M.; Kaivola, M.; Metrangolo, P.; Milani, R.; Shishido, A.; Pilati, T.; Resnati, G. Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly and Performance of Light-Responsive Supramolecular Polymers. Adv. Funct. Mater. 2012, 22, 2572–2579. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, T.; Matsuda, H.; Shiraga, T.; Kimura, T.; Kato, M.; Viswanathan, N.K.; Kumar, J.; Tripathy, S.K. Photofabrication of surface relief grating on films of azobenzene polymer with different dye functionalization. Macromolecules 2000, 33, 4220–4225. [Google Scholar] [CrossRef]
- Luong, M.H.; Nguyen, T.T.N.; Nguyen, C.T.; Ledoux-Rak, I.; Lai, N.D. Study of all-polymer-based waveguide resonant gratings and their applications for optimization of second-harmonic generation. J. Phys. D Appl. Phys. 2015, 48, 365302. [Google Scholar] [CrossRef]
- Paterson, J.; Natansohn, A.; Rochon, P.; Callender, C.; Robitaille, L. Optically inscribed surface relief diffraction gratings on azobenzene-containing polymers for coupling light into slab waveguides. Appl. Phys. Lett. 1996, 69, 3318–3320. [Google Scholar] [CrossRef]
- Gao, J.; He, Y.; Xu, H.; Song, B.; Zhang, X.; Wang, Z.; Wang, X. Azobenzene-containing supramolecular polymer films for laser-induced surface relief gratings. Chem. Mater. 2007, 19, 14–17. [Google Scholar] [CrossRef]
- Priimagi, A.; Lindfors, K.; Kaivola, M.; Rochon, P. Efficient Surface-Relief Gratings in Hydrogen-Bonded Polymer− Azobenzene Complexes. ACS Appl. Mater. Inter. 2009, 1, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Pawlicka, A.; Mattos, R.I.; Tambelli, C.E.; Silva, I.D.A.; Magon, C.J.; Donoso, J.P. Magnetic resonance study of chitosan bio-membranes with proton conductivity properties. J. Membrane Sci. 2013, 429, 190–196. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Alves, R.; Sentanin, F.; Sabadini, R.C.; Fernandes, M.; de Zea Bermudez, V.; Pawlicka, A.; Silva, M.M. Samarium (III) triflate-doped chitosan electrolyte for solid state electrochromic devices. Electrochim. Acta 2018, 267, 51–62. [Google Scholar] [CrossRef]
- Alves, R.; Sentanin, F.; Sabadini, R.C.; Pawlicka, A.; Silva, M.M. Influence of cerium triflate and glycerol on electrochemical performance of chitosan electrolytes for electrochromic devices. Electrochim. Acta 2016, 217, 108–116. [Google Scholar] [CrossRef]
- García, O.G.Z.; Oropeza-Guzmán, M.T.; Monal, W.M.A.; López-Maldonado, E.A. Design and mechanism of action of multifunctional BPE’s with high performance in the separation of hazardous metal ions from polluted water Part I: Chitosan-poly (N-vinylcaprolactam) and its derivatives. Chem. Eng. J. 2019, 359, 840–851. [Google Scholar] [CrossRef]
- Harzendorf, T.; Michaelis, D.; Flügel-Paul, T.; Bianco, A.; Oliva, E.; Zeitner, U. Surface relief gratings manufactured by lithographic means being a candidate for VLT MOONS instrument’s main dispersers. In Advances in Optical and Mechanical Technologies for Telescopes and Instrumentation III; International Society for Optics and Photonics: Austin, TX, USA, 2018; p. 1070621. [Google Scholar]
- Fiorini, C.; Prudhomme, N.; De Veyrac, G.; Maurin, I.; Raimond, P.; Nunzi, J.-M. Molecular migration mechanism for laser induced surface relief grating formation. Synth. Met. 2000, 115, 121–125. [Google Scholar] [CrossRef]
- Abdulsahib, H.T.; Taobi, A.H.; Hashem, S.S. A novel Coagulant based on Chitosan and Lignin for the Removal of Bentonite from Raw water. Adv. J. Sci. Res. 2016, 1, 1–10. [Google Scholar]
- Alves, R.; Sentanin, F.; Sabadini, R.; Pawlicka, A.; Silva, M.M. Innovative electrolytes based on chitosan and thulium for solid state applications: Synthesis, structural, and thermal characterization. J. Electroanal. Chem. 2017, 788, 156–164. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; dos Santos, D.S., Jr.; Oliveira, O.N., Jr. Interaction between cholesterol and chitosan in Langmuir monolayers. Polimeros 2005, 15, 91–94. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Jańczyk, A. Probing the modes of antibacterial activity of chitosan. Effects of pH and molecular weight on chitosan interactions with membrane lipids in Langmuir films. Biomacromolecules 2011, 12, 4144–4152. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Marine Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Wan Ngah, W.S.; Ariff, N.F.M.; Hanafiah, M.A.K.M. Preparation, Characterization, and Environmental Application of Crosslinked Chitosan-Coated Bentonite for Tartrazine Adsorption from Aqueous Solutions. Water Air Soil Pollut. 2010, 206, 225–236. [Google Scholar] [CrossRef]
- Alves, R.; de Camargo, A.; Pawlicka, A.; Silva, M. Luminescent polymer electrolytes based on chitosan and containing europium triflate. J. Rare Earth 2016, 34, 661–666. [Google Scholar] [CrossRef]
- Leones, R.; Reis, P.M.; Sabadini, R.C.; Ravaro, L.P.; Silva, I.D.A.; de Camargo, A.S.S.; Donoso, J.P.; Magon, C.J.; Esperança, J.M.S.S.; Pawlicka, A.; et al. A luminescent europium ionic liquid to improve the performance of chitosan polymer electrolytes. Electrochim. Acta 2017, 240, 474–485. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atmah, N.R.A.; Caliman, W.R.; Pawlicka, A.; Sabat, R.G.; Nunzi, J.-M. Surface Relief Grating on Chitosan-N,N-dimethyl-4-(2-pyridylazo)aniline Thin Film. Polymers 2022, 14, 791. https://doi.org/10.3390/polym14040791
Atmah NRA, Caliman WR, Pawlicka A, Sabat RG, Nunzi J-M. Surface Relief Grating on Chitosan-N,N-dimethyl-4-(2-pyridylazo)aniline Thin Film. Polymers. 2022; 14(4):791. https://doi.org/10.3390/polym14040791
Chicago/Turabian StyleAtmah, Nadiyah Rashed Al, Willian R. Caliman, Agnieszka Pawlicka, Ribal Georges Sabat, and Jean-Michel Nunzi. 2022. "Surface Relief Grating on Chitosan-N,N-dimethyl-4-(2-pyridylazo)aniline Thin Film" Polymers 14, no. 4: 791. https://doi.org/10.3390/polym14040791