Properties of Biocomposite Film Based on Whey Protein Isolate Filled with Nanocrystalline Cellulose from Pineapple Crown Leaf
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
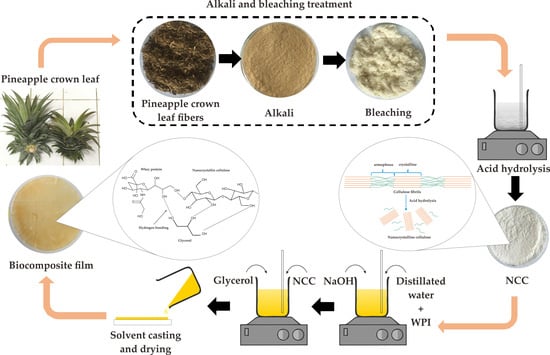
2.2. Preparation of Nanocrystalline Cellulose from Pineapple Crown Leaf
2.3. Preparation of Biocomposite Film
2.4. Physical and Mechanical Properties
2.5. Morphology and Thermal Properties of Nanocrystalline Cellulose
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties
3.1.1. Film Thickness
3.1.2. Film Transparency
3.1.3. Moisture Content
3.1.4. Water Solubility
3.1.5. Moisture Absorption
3.2. Mechanical Properties
3.3. Morphological Properties
3.3.1. Morphology
3.3.2. Surface Chemistry
3.3.3. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Bano, K.; Zaheer, M.R.; Kuddus, M. Biodegradable smart biopolymers for food packaging: Sustainable approach toward green environment. In Bio-Based Materials for Food Packaging; Springer: Berlin/Heidelberg, Germany, 2018; pp. 197–216. [Google Scholar]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide based bionanocomposites, properties and applications: A review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.G.; Yuhana, N.Y.; Zawawi, E.Z.E. Review of bioplastics as food packaging materials. AIMS Mater. Sci. 2021, 8, 166–184. [Google Scholar] [CrossRef]
- Oymaci, P.; Altinkaya, S.A. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll. 2016, 54, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Reis, M.O.; Zanela, J.; Olivato, J.; Garcia, P.S.; Yamashita, F.; Grossmann, M.V.E. Microcrystalline Cellulose as Reinforcement in Thermoplastic Starch/Poly(butylene adipate-co-terephthalate) Films. J. Polym. Environ. 2014, 22, 545–552. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Claro, P.; Neto, A.; Bibbo, A.; Mattoso, L.; Bastos, M.S.R.; Marconcini, J.M. Biodegradable blends with potential use in packaging: A comparison of PLA/chitosan and PLA/cellulose acetate films. J. Polym. Environ. 2016, 24, 363–371. [Google Scholar] [CrossRef]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132, 42004. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.; Yépiz-Gómez, M.; Plascencia-Jatomea, M.J.C.P. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Wildan, M.W.; Lubis, F.I. Fabrication and Characterization of Chitosan/Cellulose Nanocrystal/Glycerol Bio-Composite Films. Polymers 2021, 13, 1096. [Google Scholar]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M.J.F.H. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Fabrication and characterization of TiO2/whey protein isolate nanocomposite film. Food Hydrocoll. 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, C.; Fan, F. Preparation and Properties of Soy Protein Isolate/Cotton-Nanocrystalline Cellulose Films. Int. J. Polym. Sci. 2021, 2021. [Google Scholar] [CrossRef]
- Batool, M.; Abid, A.; Khurshid, S.; Bashir, T.; Ismail, M.A.; Razaq, M.A. Engineering, Quality Control of Nano-food Packing Material for Grapes (Vitis vinifera) Based on ZnO and Polylactic Acid (PLA) biofilm. Arab. J. Sci. Eng. 2021, 1–13. [Google Scholar] [CrossRef]
- Yoo, S.; Krochta, J.M. Whey protein–polysaccharide blended edible film formation and barrier, tensile, thermal and transparency properties. J. Sci. Food Agric. 2011, 91, 2628–2636. [Google Scholar] [CrossRef]
- Erdem, B.G.; Dıblan, S.; Kaya, S. Development and structural assessment of whey protein isolate/sunflower seed oil biocomposite film. Food Bioprod. Process. 2019, 118, 270–280. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sandeep, K.; Alavi, S.; Truong, V.; Gorga, R. Effect of type and content of modified montmorillonite on the structure and properties of bio-nanocomposite films based on soy protein isolate and montmorillonite. J. Food Sci. 2010, 75, N46–N56. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Amica, G.; Rácz, I.; Marcovich, N.E. Structure and properties of nanocomposite films based on sodium caseinate and nanocellulose fibers. J. Food Eng. 2011, 103, 76–83. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and characterization of the kefiran-whey protein isolate-TiO2 nanocomposite films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; Borges, S.V.; Costa, A.L.R.; Silva, E.K.; Medeiros, É.A.A.; Nilda de Fátima, F.S. Development of whey protein isolate bio-nanocomposites: Effect of montmorillonite and citric acid on structural, thermal, morphological and mechanical properties. Food Hydrocoll. 2015, 48, 179–188. [Google Scholar] [CrossRef]
- Rahmawati, C.; Aprilia, S.; Saidi, T.; Aulia, T.B.; Ahmad, I. Preparation and Characterization of Cellulose Nanocrystals from Typha sp. as a Reinforcing Agent. J. Nat. Fibers 2021, 1–14. [Google Scholar] [CrossRef]
- Aprilia, N.A.S.; Arahman, N. Properties of nanocrystalline cellulose from pineapple crown leaf waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 796, 012007. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Santos, R.M.D.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crop. Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–Soy hulls. Ind. Crop. Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Rosa, M.; Medeiros, E.; Malmonge, J.; Gregorski, K.; Wood, D.; Mattoso, L.; Glenn, G.; Orts, W.; Imam, S. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Rahmawati, C.; Aprilia, S.; Saidi, T.; Aulia, T.B. Current development of geopolymer cement with nanosilica and cellulose nanocrystals. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Aprilia, N.A.S.; Davoudpour, Y.; Zulqarnain, W.; Khalil, H.A.; Hazwan, C.M.; Hossain, M.S.; Dungani, R.; Fizree, H.M.; Zaidon, A.; Haafiz, M.K.M. Physicochemical Characterization of Microcrystalline Cellulose Extracted from Kenaf Bast. Bioresources 2016, 11, 3875–3889. [Google Scholar] [CrossRef] [Green Version]
- Aprilia, N.; Mulyati, S.; Alam, P.; Ambarita, A. Characterization nano crystalline cellulose from sugarcane baggase for reinforcement in polymer composites: Effect of formic acid concentrations. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Zhang, P.P.; Tong, D.S.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Yu, W.H.; Wang, H.; Zhou, C.H. Effects of acid treatments on bamboo cellulose nanocrystals. Asia-Pac. J. Chem. Eng. 2014, 9, 686–695. [Google Scholar] [CrossRef]
- Silverio, H.A.; Neto, W.P.F.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crop. Prod. 2013, 44, 427–436. [Google Scholar] [CrossRef]
- Schmid, M. Properties of cast films made from different ratios of whey protein isolate, hydrolysed whey protein isolate and glycerol. Materials 2013, 6, 3254–3269. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Microfibrillated cellulose addition improved the physicochemical and bioactive properties of biodegradable films based on soy protein and clove essential oil. Food Hydrocoll. 2018, 79, 416–427. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Kadivar, M. Properties of whey protein isolate nanocomposite films reinforced with nanocellulose isolated from oat husk. Int. J. Biol. Macromol. 2016, 91, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, J.; Zhang, Q.; Zhao, G.; Heng, L.; Chen, D.; Liu, D. Preparation of cellulose nanocrystals from Humulus japonicus stem and the influence of high temperature pretreatment. Carbohydr. Polym. 2017, 164, 284–293. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Chang, P.R.; Anderson, D.P.; Huneault, M.A. Pea starch-based composite films with pea hull fibers and pea hull fiber-derived nanowhiskers. Polym. Eng. Sci. 2009, 49, 369–378. [Google Scholar] [CrossRef]
- Ekielski, A.; Żelaziński, T.; Mishra, P.K.; Skudlarski, J. Properties of Biocomposites Produced with Thermoplastic Starch and Digestate: Physicochemical and Mechanical Characteristics. Materials 2021, 14, 6092. [Google Scholar] [CrossRef] [PubMed]
- Majer, Z.; Hutař, P.; Nahlik, L. Determination of the effect of interphase on the fracture toughness and stiffness of a particulate polymer composite. Mech. Compos. Mater. 2013, 49, 475–482. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J.-W. Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 2016, 135, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-G.; Qi, X.-M.; Guan, Y.; Peng, F.; Yao, C.-L.; Sun, R.-C. High strength hemicellulose-based nanocomposite film for food packaging applications. ACS Sustain. Chem. Eng. 2016, 4, 1985–1993. [Google Scholar] [CrossRef]
- Karimi, S.; Tahir, P.M.; Dufresne, A.; Karimi, A.; Abdulkhani, A. A comparative study on characteristics of nanocellulose reinforced thermoplastic starch biofilms prepared with different techniques. Nord. Pulp Pap. Res. J. 2014, 29, 41–45. [Google Scholar] [CrossRef]






| Biopolymer | Advantage Properties | Disadvantage Properties | Application | References |
|---|---|---|---|---|
| Starch | Good barrier against oxygen and good elongation | Poor mechanical and water barrier properties | Food packaging, biomaterial, composite film | [3,7] |
| Cellulose | Non-toxicity, biodegradability, and chemically stable | Poor water barrier properties and limited soluble with solvent | Packaging and biodegradable film | [8,9] |
| chitosan | Non-toxicity, good compatible and good film-forming | Poor barrier properties and insoluble in water | Antimicrobial agent, hybrid composite film | [10,11,12,13] |
| Whey protein | Excellent oxygen barrier, biodegradability, and good film-forming | Poor mechanical properties and high sensitivity to humidity | Biocomposite film, hybrid composite film | [14,15,16] |
| PLA | Biodegradability, good physical and mechanical properties | Low thermal properties | Food packaging and biomaterial | [9,17] |
| Film | Thickness (mm) |
|---|---|
| WPI | 0.081 ± 0.02 ab |
| WPI/NCC 1% | 0.112 ± 0.01 d |
| WPI/NCC 3% | 0.117 ± 0.01 d |
| WPI/NCC 5% | 0.066 ± 0.01 a |
| WPI/NCC 7% | 0.086 ± 0.02 ab |
| WPI/NCC 10% | 0.091 ± 0.01 c |
| Film | Wavelength (nm) | |||||
|---|---|---|---|---|---|---|
| 300 | 400 | 500 | 600 | 700 | 800 | |
| WPI | 0 | 5.529 ± 0.10 | 10.803 ± 0.10 | 13.537 ± 0.09 | 15.417 ± 0.11 | 15.844 ± 0.46 |
| WPI/NCC 1% | 0 | 1.354 ± 0.09 | 2.160 ± 0.11 | 2.661 ± 0.85 | 2.941 ± 0.70 | 3.198 ± 0.70 |
| WPI/NCC 3% | 0 | 0.939 ± 0.58 | 2.136 ± 0.30 | 2.990 ± 0.00 | 3.479 ± 0.02 | 3.845 ± 0.11 |
| WPI/NCC 5% | 0 | 0.927 ± 0.00 | 2.380 ± 0.45 | 3.759 ± 0.23 | 4.833 ± 0.15 | 5.737 ± 0.15 |
| WPI/NCC 7% | 0 | 0.854 ± 0.07 | 1.989 ± 0.16 | 2.783 ± 0.31 | 3.259 ± 0.09 | 3.613 ± 0.39 |
| WPI/NCC 10% | 0 | 0.781 ± 0.09 | 1.330 ± 0.12 | 1.794 ± 0.11 | 2.062 ± 0.35 | 2.258 ± 0.26 |
| Film | Moisture Content (%) | Water Solubility (%) | Moisture Absorption (%) |
|---|---|---|---|
| WPI | 0.050 ± 0.02 c | 92.174 ± 5.48 d | 5.051 ± 0.31 d |
| WPI/NCC 1% | 0.046 ± 0.01 bc | 88.245 ± 1.60 d | 5.263 ± 0.17 d |
| WPI/NCC 3% | 0.032 ± 0.00 ab | 83.750 ± 3.68 cd | 4.310 ± 0.19 c |
| WPI/NCC 5% | 0.028 ± 0.00 ab | 82.353 ± 5.20 c | 4.082 ± 0.09 c |
| WPI/NCC 7% | 0.027 ± 0.01 ab | 75.806 ± 2.72 b | 2.889 ± 0.89 b |
| WPI/NCC 10% | 0.180 ± 0.01 a | 65.455 ± 1.78 a | 1.695 ± 0.16 a |
| Film | Tonset (°C) | T10% (°C) | T50% (°C) | Tmax (°C) |
|---|---|---|---|---|
| WPI | 261 | 294 | 348 | 352 |
| WPI/NCC 1% | 265 | 297 | 340 | 350 |
| WPI/NCC 3% | 268 | 295 | 348 | 356 |
| WPI/NCC 5% | 273 | 297 | 335 | 355 |
| WPI/NCC 7% | 273 | 297 | 339 | 347 |
| WPI/NCC 10% | 282 | 302 | 331 | 348 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitriani, F.; Aprilia, S.; Arahman, N.; Bilad, M.R.; Suhaimi, H.; Huda, N. Properties of Biocomposite Film Based on Whey Protein Isolate Filled with Nanocrystalline Cellulose from Pineapple Crown Leaf. Polymers 2021, 13, 4278. https://doi.org/10.3390/polym13244278
Fitriani F, Aprilia S, Arahman N, Bilad MR, Suhaimi H, Huda N. Properties of Biocomposite Film Based on Whey Protein Isolate Filled with Nanocrystalline Cellulose from Pineapple Crown Leaf. Polymers. 2021; 13(24):4278. https://doi.org/10.3390/polym13244278
Chicago/Turabian StyleFitriani, Fitriani, Sri Aprilia, Nasrul Arahman, Muhammad Roil Bilad, Hazwani Suhaimi, and Nurul Huda. 2021. "Properties of Biocomposite Film Based on Whey Protein Isolate Filled with Nanocrystalline Cellulose from Pineapple Crown Leaf" Polymers 13, no. 24: 4278. https://doi.org/10.3390/polym13244278