Cellulose Nanocrystals as Template for Improving the Crystallinity of Two-Dimensional Covalent Organic Framework Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNCs
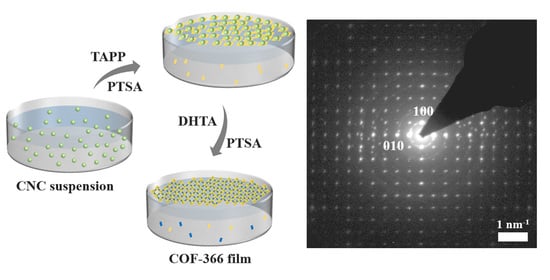
2.3. Synthesis of 2D COF Films
2.4. Instruments and Measurements
3. Results and Discussion
3.1. Raman Spectra and FTIR Spectra of the Films with Different CNC Amounts
3.2. The Influence of CNC Amount on Morphology
3.3. Spectroscopic Characterization of COF-366 Film with 100 μL CNCs
3.4. TEM and SAED Characterization of COF-366 Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Sun, C.; Feng, X. Crystallinity and stability of covalent organic framework. Sci. China Chem. 2020, 63, 1367–1390. [Google Scholar] [CrossRef]
- Guan, X.Y.; Li, H.; Ma, Y.C.; Xue, M.; Fang, Q.R.; Yan, Y.S.; Valtchev, V.; Qiu, S.L. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 2019, 11, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.C.; Shi, Z.Q.; Wang, X.Y.; Li, Z.F.; Li, G. Proton conductive covalent organic frameworks. Coord. Chem. Rev. 2020, 422, 213465. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Zhuang, W.C.; Shi, X.Q.; Du, X.H. Properties of metal-doped covalent organic frameworks and their interactions with sulfur dioxide. J. Chem. N. Y. 2018, 2018, 9321347. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Yin, S.; Lin, G.Q.; Ma, T.Q.; Meng, Y.; Sun, J.L.; Wang, W. Isostructural three-dimensional covalent organic frameworks. Angew. Chem. Int. Ed. 2019, 58, 9770–9775. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, Q.Y.; Ma, Y.C.; Guan, X.Y.; Xue, M.; Fang, Q.R.; Yan, Y.S.; Valtchev, V.; Qiu, S.L. Three-dimensional covalent organic frameworks with dual linkages for bifunctional cascade catalysis. J. Am. Chem. Soc. 2016, 138, 14783–14788. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, N.; Ma, L.L. Recent advances in covalent organic frameworks for catalysis. Chem. Asian J. 2020, 15, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, J.; Jiang, D.L. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D.L. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 2009, 121, 3243–3253. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Xu, Y.X.; Pang, H. Two-dimensional MOF and COF nanosheets: Synthesis and applications in electrochemistry. Chem. Eur. J. 2020, 26, 6402–6422. [Google Scholar] [CrossRef]
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X.F.; et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 2011, 23, 4094–4097. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L.X.; Wang, H.J.; Xu, Z.; Zhao, Y.M.; Luo, Y.; Nasir, N.; Song, Y.M.; Wu, H.; Pan, F.S.; et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019, 10, 2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Hu, Y.; Ortiz, M.; Huang, S.; Ge, Y.; Zhang, W. Confined growth of ordered organic frameworks at an interface. Chem. Soc. Rev. 2020, 49, 4637–4666. [Google Scholar] [CrossRef]
- Zhang, B.; Song, X.Y.; Li, Y.S.; Li, Y.; Peng, Z.X.; Ye, L.; Chen, L. 2D Covalent organic framework thin films via interfacial self-polycondensation of an A2B2 type monomer. Chem. Commun. 2020, 56, 3253–3256. [Google Scholar] [CrossRef] [PubMed]
- Sahabudeen, H.; Qi, H.Y.; Glatz, B.A.; Tranca, D.; Dong, R.H.; Hou, Y.; Zhang, T.; Kuttner, C.; Lehnert, T.; Seifert, G.; et al. Wafer-sized multifunctional polyimine-based two-dimensional conjugated polymers with high mechanical stiffness. Nat. Commun. 2016, 7, 13461. [Google Scholar] [CrossRef]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent organic frameworks: Chemistry beyond the structure. J. Am. Chem. Soc. 2019, 141, 1807–1822. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Q.Y.; Kang, H.; Chen, R.Z.; Liu, Y.Q.; Wei, D.C. Self-controlled growth of covalent organic frameworks by repolymerization. Chem. Mater. 2020, 32, 5634–5640. [Google Scholar] [CrossRef]
- Evans, A.M.; Parent, L.R.; Flanders, N.C.; Bisbey, R.P.; Vitaku, E.; Kirschner, M.S.; Schalle, R.D.; Chen, L.X.; Gianneschi, N.C.; Dichtel, W.R. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 2018, 361, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, D.W.; Sun, C.; Castano, I.; Flanders, N.C.; Evans, A.M.; Vitaku, E.; McLeod, D.C.; Lambeth, R.H.; Chen, L.X.; Gianneschi, N.C.; et al. Acid Exfoliation of Imine-linked Covalent Organic Frameworks enables solution processing into crystalline thin films. Angew. Chem. Int. Ed. 2020, 59, 5165–5171. [Google Scholar] [CrossRef] [PubMed]
- Sahabudeen, H.; Qi, H.Y.; Ballabio, M.; Položij, M.; Olthof, S.; Shivhare, R.; Jing, Y.; Park, S.W.; Liu, K.J.; Zhang, T.; et al. Highly crystalline and semiconducting imine-based two-dimensional. Angew. Chem. Int. Ed. 2020, 59, 6028–6036. [Google Scholar] [CrossRef] [Green Version]
- Gal, A.; Wirth, R.; Barkay, Z.; Eliaz, N.; Scheffel, A.; Faivre, D. Templated and self-limiting calcite formation directed by coccolith organic macromolecules. Chem. Commun. 2017, 53, 7740–7743. [Google Scholar] [CrossRef] [Green Version]
- Giese, M.; Blusch, L.K.; Khan, M.K.; Hamad, W.Y.; MacLachlan, M.J. Responsive mesoporous photonic cellulose films by supramolecular cotemplating. Angew. Chem. Int. Ed. 2014, 126, 9026–9030. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Li, W.R.; Li, W.; Wang, X.J.; Tang, Z.Y.; Zhang, S.X.A.; Xu, Y. Uncovering the circular polarization potential of chiral photonic cellulose films for photonic applications. Adv. Mater. 2018, 30, 1705948. [Google Scholar] [CrossRef]
- Lin, W.S.; Hong, W.; Sun, L.; Yu, D.; Yu, D.S.; Chen, X.D. Bioinspired mesoporous chiral nematic graphitic carbon nitride photocatalysts modulated by polarized light. ChemSusChem 2018, 11, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lokanathan, A.R.; Uddin, K.M.A.; Rojas, O.J.; Laine, J. Cellulose nanocrystal-mediated synthesis of silver nanoparticles: Role of sulfate groups in nucleation phenomena. Biomacromolecules 2014, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Pei, A.H.; Zhou, Q.; Berglund, L.A. Functionalized cellulose nanocrystals as biobased nucleation agents in poly(l-lactide) (PLLA)–crystallization and mechanical property effects. Compos. Sci. Technol. 2010, 70, 815–821. [Google Scholar] [CrossRef]
- Jiang, L.; Lv, P.; Ma, P.M.; Bai, H.Y.; Dong, W.F.; Chen, M.Q. Stereocomplexation kinetics of enantiomeric poly(l-lactide)/poly(d-lactide) blends seeded by nanocrystalline cellulose. RSC. Adv. 2015, 5, 71115–71119. [Google Scholar] [CrossRef]
- Richardson, J.J.; Tardy, B.L.; Guo, J.L.; Liang, K.; Rojas, O.J.; Ejima, H. Continuous metal-organic framework biomineralization on cellulose nanocrystals: Extrusion of functional composite filaments. ACS. Sustain. Chem. Eng. 2019, 7, 6287–6294. [Google Scholar] [CrossRef]
- Lu, Z.H.; Liu, M.F.; Gao, Q.W.; Yang, D.Q.; Zhang, Z.S.; Xiong, X.P.; Jiang, Y.; Liu, X.Y. Design of heterogeneous nuclei composed of uniaxial cellulose nanocrystal assemblies for epitaxial growth of poly(ε-caprolactone). Macromolecules 2017, 50, 3355–3364. [Google Scholar] [CrossRef]
- Chen, J.Y.; Xu, L.R.; Lin, X.F.; Chen, R.L.; Hong, W.; Zheng, Z.K.; Chen, X.D. Self-healing responsive chiral photonic film for sensing and encoding. J. Mater. Chem. C 2018, 6, 7767–7775. [Google Scholar] [CrossRef]
- Leng, J.X.; Li, G.H.; Ji, X.X.; Yuan, Z.W.; Fu, Y.J.; Li, H.G.; Qin, M.H.; Moehwald, H. Flexible latex photonic films with tunable structural colors templated by cellulose nanocrystals. J. Mater. Chem. C 2018, 6, 2396–2406. [Google Scholar] [CrossRef]
- Liu, X.; Shi, S.W.; Li, Y.N.; Forth, J.; Wang, D.; Russell, T.P. Liquid tubule formation and stabilization using cellulose nanocrystal surfactants. Angew. Chem. Int. Ed. 2017, 56, 12594–12598. [Google Scholar] [CrossRef]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Kunjattu, H.S.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective molecular sieving in self-standing porous covalent-organic-framework membranes. Adv. Mater. 2017, 29, 1603945. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.Y.; Shao, F.; Szczerbiński, J.; McCaffrey, R.; Zenobi, R.; Jin, Y.H.; Schlüter, A.D.; Zhang, W. Synthesis of a two-dimensional covalent organic monolayer through dynamic imine chemistry at the air/water interface. Angew. Chem. Int. Ed. 2016, 55, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.L.; Han, S.; Peng, D.L.; Wang, H.L.; Yang, J.; Zhao, P.; Ye, X.J.; Dong, X.; Zheng, Y.Y.; Zheng, N.; et al. Nanoporous and highly thermal conductive thin film of single-crystal covalent organic frameworks ribbons. J. Am. Chem. Soc. 2021, 143, 3927–3933. [Google Scholar] [CrossRef] [PubMed]
- Royuela, S.; Almarza, J.; Mancheno, M.J.; Perez-Flores, J.C.; Michel, E.G.; Ramos, M.M.; Zamora, F.; Ocon, P.; Segura, J.L. Synergistic effect of covalent bonding and physical encapsulation of sulfur in the pores of a microporous COF to improve cycling performance in Li-S batteries. Chem. Eur. J. 2019, 25, 12394–12404. [Google Scholar] [CrossRef] [Green Version]
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of chemical stability and crystallinity in porphyrin-containing covalent organic frameworks by intramolecular hydrogen bonds. Angew. Chem. Int. Ed. 2013, 52, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Pradines, V.; Bijani, C.; Stigliani, J.L.; Blanzat, M.; Rico-Lattes, I.; Pratviel, G. Cationic porphyrin-anionic surfactant mixtures for the promotion of self-organized 1:4 ion pairs in water with strong aggregation properties. ChemPhysChem 2015, 16, 3877–3885. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.L.; Peng, C.S.; Song, S.X.; Lopez-Valdivieso, A. Lime mortars-the role of carboxymethyl cellulose on the crystallization of calcium carbonate. Constr. Build. Mater. 2018, 168, 169–177. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, R. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan–Based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Förster, S.; Schmidt, M. Polyelectrolytes in solution. Adv. Polym. Sci. 2005, 120, 51–133. [Google Scholar]
- Qi, H.Y.; Sahabudeen, H.; Liang, B.K.; Položij, M.; Addicoat, M.A.; Gorelik, T.E.; Hambsch, M.; Mundszinger, M.; Park, S.W.; Lotsch, B.V.; et al. Near-atomic-scale observation of grain boundaries in a layer-stacked two-dimensional polymer. Sci. Adv. 2020, 6, 5976. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ou, Z.; Liang, B.; Yang, J.; Chen, R.; Qi, H.; Kaiser, U.; Hong, W.; Chen, X.; Du, L.; et al. Cellulose Nanocrystals as Template for Improving the Crystallinity of Two-Dimensional Covalent Organic Framework Films. Polymers 2021, 13, 1561. https://doi.org/10.3390/polym13101561
Li Y, Ou Z, Liang B, Yang J, Chen R, Qi H, Kaiser U, Hong W, Chen X, Du L, et al. Cellulose Nanocrystals as Template for Improving the Crystallinity of Two-Dimensional Covalent Organic Framework Films. Polymers. 2021; 13(10):1561. https://doi.org/10.3390/polym13101561
Chicago/Turabian StyleLi, Yue, Zhaowei Ou, Baokun Liang, Jing Yang, Ruilian Chen, Haoyuan Qi, Ute Kaiser, Wei Hong, Xudong Chen, Liangwei Du, and et al. 2021. "Cellulose Nanocrystals as Template for Improving the Crystallinity of Two-Dimensional Covalent Organic Framework Films" Polymers 13, no. 10: 1561. https://doi.org/10.3390/polym13101561