Poly(etheretherketone)/Poly(ethersulfone) Blends with Phenolphthalein: Miscibility, Thermomechanical Properties, Crystallization and Morphology
Abstract
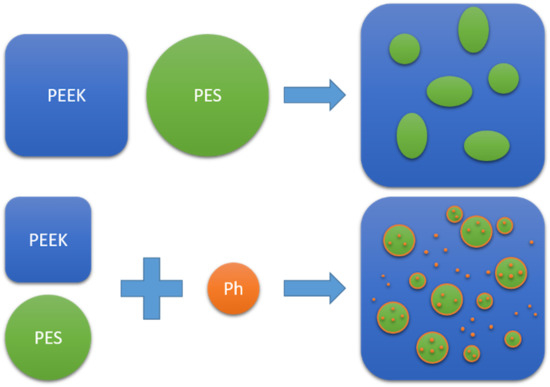
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Blends Preparation
2.2.2. Preparation of Samples
2.2.3. Experimental Methods
3. Results and Discussion
3.1. PEEK/PES Blends
3.1.1. Miscibility by DSC and DMTA
3.1.2. Crystallization of Blends
3.2. PEEK/PES Blends with Phenolphthalein
3.2.1. Miscibility by DSC and DMTA
3.2.2. Crystallization of Blends
3.2.3. Morphological Analysis by SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, J.E.; Robeson, L.M. Miscible blends of poly(aryl ether ketone)s and polyetherimides. J. Appl. Polym. Sci. 1988, 35, 1877–1891. [Google Scholar] [CrossRef]
- Goodwin, A.A.; Hay, J.N.; Mouledous, G.A.C.; Biddlestone, F. A compatible blend of poly(ether ether ketonex)(PEEK) and poly(ether imide)(Ultem 1000). In Integration of Fundamental Polymer Science and Technology-5; Springer: Limburg, The Netherlands, 1991; pp. 44–50. [Google Scholar]
- Crevecoeur, G.; Groeninckx, G. Binary blends of poly(ether ether ketone) and poly(ether imide): Miscibility, crystallization behavior and semicrystalline morphology. Macromolecules 1991, 24, 1190–1195. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Sauer, B.B. Glass transition, crystallization, and morphology relationships in miscible poly(aryl ether ketones) and poly(ether imide) blends. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 901–915. [Google Scholar] [CrossRef]
- Ramani, R.; Alam, S. Composition optimization of PEEK/PEI blend using model-free kinetics analysis. Thermochim. Acta 2010, 511, 179–188. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Wu, Z.; Tang, X.; Jiang, B. Study on the compatibility of the blend of poly(aryl ether ether ketone) with poly(aryl ether sulfone). J. Appl. Polym. Sci. 1990, 41, 2649–2654. [Google Scholar] [CrossRef]
- Ni, Z. The preparation, compatibility and structure of PEEK–PES blends. Polym. Adv. Technol. 1994, 5, 612–614. [Google Scholar] [CrossRef]
- Malik, T.M. Thermal and mechanical characterization of partially miscible blends of poly(ether ether ketone) and polyethersulfone. J. Appl. Polym. Sci. 1992, 46, 303–310. [Google Scholar] [CrossRef]
- Arzak, A.; Eguiazabal, J.I.; Nazabal, J. Phase behaviour and mechanical properties of poly(ether ether ketone)-poly(ether sulphone) blends. J. Mater. Sci. 1991, 26, 5939–5944. [Google Scholar] [CrossRef]
- Noolandi, J. Multiblock copolymers as polymeric surfactants: Are “pancakes” better than “dumbbells”? Macromol. Theory Simul. 1992, 1, 295–298. [Google Scholar] [CrossRef]
- Hoffmann, T.; Pospiech, D.; Häussler, L.; Pötschke, P.; Reuter, U.; Werner, P.; Sandler, J.K.W.; Döring, M.; Altstädt, V. Properties of segmented block copolymers in PEEK/PSU blends. High Perform. Polym. 2008, 20, 601–614. [Google Scholar] [CrossRef]
- Mitschang, P.; Blinzler, M.; Wöginger, A. Processing technologies for continuous fibre reinforced thermoplastics with novel polymer blends. Compos. Sci. Technol. 2003, 63, 2099–2110. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, H. Morphology, crystallization kinetics and melting behaviour of the blends of poly(ether ether ketone) with poly(ether sulfone with cardo side group). Polymer 1993, 34, 4032–4037. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, T.; Bo, S.; Xu, J. Novel macrocyclic precursors of phenolphthalein poly(arylene ether ketone) and poly(arylene ether sulfone): Synthesis and polymerization. Macromolecules 1997, 30, 7345–7347. [Google Scholar] [CrossRef]
- Song, G.; Li, J.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Preparation and characterization of PES-C/PVPP nanofibrous composite membranes via soultion-blowing. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; IOP Publishing: Bristol, UK, 2018; pp. 1–7. [Google Scholar]
- Garcia-Leiner, M.; Reitman, M.T.F.; El-Hibri, M.J.; Roeder, R.K. Structure-property relationships in commercial polyetheretherketone resins. Polym. Eng. Sci. 2017, 57, 955–964. [Google Scholar] [CrossRef]
- BASF. Ultrason, High-Performance Thermoplastics for Membranes; BASF: Ludwigshafen, Germany, 2018. [Google Scholar]
- Blundell, D.J.; Osborn, B.N. The morphology of poly(aryl-ether-ether-ketone). Polymer 1983, 24, 953–958. [Google Scholar] [CrossRef]
- Wetton, R.E.; Corish, P.J. DMTA studies of polymer blends and compatibility. Polym. Test. 1988, 8, 303–312. [Google Scholar] [CrossRef]
- Illers, K.H.; Breuer, H. Molecular motions in polyethylene terephthalate. J. Colloid Sci. 1963, 18, 1–31. [Google Scholar] [CrossRef]
- Greiner, S.; Wudy, K.; Lanzl, L.; Drummer, D. Selective laser sintering of polymer blends: Bulk properties and process behavior. Polym. Test. 2017, 64, 136–144. [Google Scholar] [CrossRef]
- Nandan, B.; Kandpal, L.D.; Mathur, G.N. Poly(ether ether ketone)/poly(aryl ether sulphone) blends: Thermal degradation behaviour. Eur. Polym. J. 2003, 39, 193–198. [Google Scholar] [CrossRef]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Lipson, J.E.G. Global and local views of the glass transition in mixtures. Macromolecules 2020, 53, 7219–7223. [Google Scholar] [CrossRef]
- Attwood, T.E.; Dawson, P.C.; Freeman, J.L.; Hoy, L.R.J.; Rose, J.B.; Staniland, P.A. Synthesis and properties of polyaryletherketones. Polymer 1981, 22, 1096–1103. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, H.C.; Chen, T.L. The Synthetic Polyether Sulphone that Has the Phthalein Side Group of Single Stage Method. CN Patent 85101721, 1 April 1985. [Google Scholar]
- Guo, R.; Lane, O.; VanHouten, D.; McGrath, J.E. Synthesis and characterization of phenolphthalein-based poly(arylene ether sulfone) hydrophilic−hydrophobic multiblock copolymers for proton exchange membranes. Ind. Eng. Chem. Res. 2010, 49, 12125–12134. [Google Scholar] [CrossRef]
- Scobbo, J.J. Dynamic mechanical analysis of compatibilized polymer blends. Polym. Test. 1991, 10, 279–290. [Google Scholar] [CrossRef]
- Nandan, B.; Kandpal, L.D.; Mathur, G.N. Polyetherether ketone/polyarylethersulfone blends: Thermal and compatibility aspects. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1407–1424. [Google Scholar] [CrossRef]
- Chen, H.L.; Porter, R.S. Phase and crystallization behavior of solution-blended poly(ether ether ketone) and poly(ether imide). Polym. Eng. Sci. 1992, 32, 1870–1875. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]















| Polymer | Melt Flow Index (g·(10 min)−1) | Molecular Weight (g·mol−1) | Shear Viscosity (Pa·s) | Density (g·cm−3) |
|---|---|---|---|---|
| PEEK 450G | 5 | 98,000 [16] | 5000 | 1.30 |
| Ultrason E1010 | 150 | 35,000 [17] | 300 | 1.37 |
| Ultrason E3010 | 35 | 58,000 [17] | 1500 | 1.37 |
| Polyetheretherketone (PEEK) | Polyethersulfone (PES) | Phenolphthalein | ||
|---|---|---|---|---|
| 450G | 1010G | 3010G | Phph | |
| (wt %) | (wt %) | (wt %) | (wt %) | |
| vPEEK | 100 | |||
| vPES | 100 | 100 | ||
| PEEK/PES | 100 | 0 | - | |
| 90 | 10 | - | ||
| 80 | 20 | - | ||
| 70 | 30 | - | ||
| 0 | 100 | - | ||
| 100 | - | 0 | ||
| 90 | - | 10 | ||
| 80 | - | 20 | ||
| 70 | - | 30 | ||
| 0 | - | 100 | ||
| PEEK/PES/Phph | 100 | 0 | - | 10 |
| 90 | 10 | - | 10 | |
| 80 | 20 | - | 10 | |
| 70 | 30 | - | 10 | |
| 0 | 100 | - | 10 | |
| 100 | - | 0 | 10 | |
| 90 | - | 10 | 10 | |
| 80 | - | 20 | 10 | |
| 70 | - | 30 | 10 | |
| 0 | - | 100 | 10 | |
| PEEK/PES Blends | Glass Transition of PEEK (°C) | Glass Transition of PES (°C) | |
|---|---|---|---|
| vPEEK | 147 | ||
| PEEK 450G/ PES 1010G | 100/0 | 159 | - |
| 90/10 | 159 | 228 | |
| 80/20 | 161 | 229 | |
| 70/30 | 160 | 230 | |
| 0/100 | - | 228 | |
| vPES | 232 | ||
| vPEEK | 147 | ||
| PEEK 450G/ PES 3010G | 100/0 | 159 | - |
| 90/10 | 158 | 233 | |
| 80/20 | 159 | 233 | |
| 70/30 | 158 | 233 | |
| 0/100 | - | 223 | |
| vPES | 236 |
| PEEK/PES/Phph Blends | Glass Transition of PEEK (°C) | Glass Transition of PES (°C) | |
|---|---|---|---|
| PEEK 450G/ PES 1010G/ Phph | 100/0/10 | 151 | - |
| 90/10/10 | 150 | 173 | |
| 80/20/10 | 153 | 179 | |
| 70/30/10 | 154 | 182 | |
| 0/100/10 | - | 191 | |
| PEEK 450G/ PES 3010G/ Phph | 100/0/10 | 151 | - |
| 90/10/10 | 149 | 174 | |
| 80/20/10 | 150 | 176 | |
| 70/30/10 | 155 | 185 | |
| 0/100/10 | - | 195 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korycki, A.; Garnier, C.; Abadie, A.; Nassiet, V.; Sultan, C.T.; Chabert, F. Poly(etheretherketone)/Poly(ethersulfone) Blends with Phenolphthalein: Miscibility, Thermomechanical Properties, Crystallization and Morphology. Polymers 2021, 13, 1466. https://doi.org/10.3390/polym13091466
Korycki A, Garnier C, Abadie A, Nassiet V, Sultan CT, Chabert F. Poly(etheretherketone)/Poly(ethersulfone) Blends with Phenolphthalein: Miscibility, Thermomechanical Properties, Crystallization and Morphology. Polymers. 2021; 13(9):1466. https://doi.org/10.3390/polym13091466
Chicago/Turabian StyleKorycki, Adrian, Christian Garnier, Amandine Abadie, Valerie Nassiet, Charles Tarek Sultan, and France Chabert. 2021. "Poly(etheretherketone)/Poly(ethersulfone) Blends with Phenolphthalein: Miscibility, Thermomechanical Properties, Crystallization and Morphology" Polymers 13, no. 9: 1466. https://doi.org/10.3390/polym13091466