Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review
Abstract
:1. Introduction
2. Natural Plant Fibers
| Advantages | Disadvantages |
|---|---|
| Less expensive | Lower mechanical properties (especially impact strength) |
| Lower weight | Higher moisture absorption |
| Higher flexibility | Lower durability |
| Renewable | Poor fire resistance |
| Biodegradable | Variation in quality |
| Good thermal and sound insulation | Restricted maximum processing temperature |
| Eco-friendly | Poor microbial resistance |
| Nontoxic | Low thermal resistance |
| Lower energy consumption | Demand and supply cycles |
| No residues when incinerated | |
| No skin irritations |

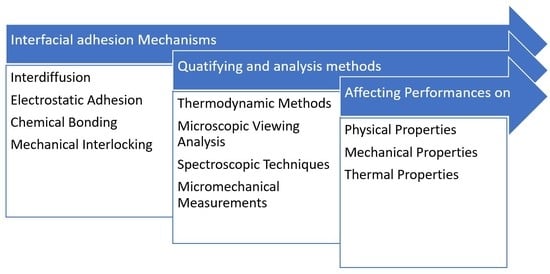
3. Interface Mechanisms
3.1. Physical Adhesion
3.2. Electrostatic Adhesion
3.3. Chemical Adhesion
3.4. Mechanical Interlocking
4. Quantifying and Analysis of Interfacial Adhesion Condition
4.1. Thermodynamic Methods
4.2. Microscopic Viewing Analysis
4.3. Spectroscopic Techniques
| Before Treatment | Raw Fiber | After Treatment | Treated Fiber | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose (wt%) | Hemicellulose (wt%) | Lignin (wt%) | Wax (wt%) | Cellulose (wt%) | Hemicellulose (wt%) | Lignin (wt%) | Wax (wt%) | |||
| Agave Americana Fibers | 68.54 | 18.41 | 6.08 | 0.56 | Alkaline | 78.65 | 8.47 | 4.65 | 0.46 | [86] |
| Stearic Acid | 81.65 | 6.31 | 3.43 | 0.37 | ||||||
| Benzoyl peroxide | 80.26 | 7.42 | 4.33 | 0.42 | ||||||
| Potassium permanganate | 79.78 | 6.67 | 4.10 | 0.22 | ||||||
| Banyan tree fibers | 67.32 | 13.46 | 15.62 | 0.81 | 5% Alkaline | 70.4 | 10.74 | 12.7 | 0.69 | [87] |
| Pennisetum orientale grass | 60.3 | 16 | 12.45 | 1.9 | HCI acid | 56.1 | 7 | 5.4 | 0.3 | [88] |
| Alkaline | 66.7 | 10.3 | 8.7 | 0.7 | ||||||
| Banana fibers | 25.51 | 2.13 | 42.50 | - | KOH | 34.24 | 5.82 | 2.72 | - | [89] |
| NaOH | 46.46 | 7.43 | 5.82 | - | ||||||
| Ramie fibers | 73.60 | 13.81 | 1.33 | 0.82 | Alkali | 90.62 | 1.04 | 0.89 | 0 | [90] |
| Peroxide | 87.43 | 9.41 | 0.47 | 0.10 | ||||||
| Peroxide and isopropyl alcohol | 91.82 | 5.23 | 0.28 | 0.05 | ||||||
| Flax fibers | 79.56 | 8.76 | - | - | NaOH/ethanol | 87.81 | 7.48 | - | - | [91] |
| EFB fibers | 53.37 | 19.88 | 10.74 | - | 0.8% NaOH | 58.93 | 22.75 | 7.03 | - | [92] |
| 0.8% Acetic acid | 63.21 | 16.30 | 7.45 | - | ||||||
| Band Position, cm−1 | Assignment |
|---|---|
| 3550–3650 | O–H stretching in free or weakly H-bonded hydroxyls |
| 3200–3400 | O–H stretching in H-bonded hydroxyls |
| 2840–2940 | C–H stretching region |
| 2725 | Overtone of interacting C–O stretch and O–H deformation |
| 2568 | Overtone of interacting C–O stretch and O–H deformation |
| 1720–1740 | C=O stretching in carbonyl |
| 1625–1660 | Adsorbed water molecules in non-crystalline cellulose |
| ~1600 | Aromatic skeleton ring vibration and vibrations owing to adsorbed water |
| ~1505 | Aromatic skeleton ring vibration |
| 1450–1475 | C–H deformation and CH2 (sym.) + OH deformation |
| 1400–1430 | C–H deformation (methoxyl group in lignin) |
| ~1370 | C–H deformation (symmetric) |
| ~1327 | Syringyl ring breathing with C–O stretching (lignin) and CH2wagging in cellulose |
| 1250–1260 | Guaiacyl ring breathing with C–O stretching (lignin) |
| 1240–1245 | C–O bond of the acetyl group in xylan and hemicellulose |
| ~1230 | Phenolic O–H deformation (lignin)–syringyl structure |
| 1160–1230 | C–O stretching of ester groups |
| 1150–1160 | C–O–C stretching (anti-symmetrical) in cellulose and aromatic C–H CH2 wagging in cellulose |
| 1098–1120 | Skeletal vibration involving C–O stretching of the β-glycosidic linkages |
| ~1060 | C–OH stretching vibration |
| 1036 | Aromatic C–H in plane deformation, guaiacyl and C–O deformation primary alcohol in lignin and C–O stretching in cellulose |
| 1003 | Skeletal vibration and C–O stretching in cellulose |
| 890–900 | Antisymmetrical stretching owing to b linkage in cellulose |
| 830 | Aromatic C–H out of plane vibration owing to lignin |
4.4. Micromechanical Measurements
5. Importance of Interfacial Adhesion Condition on Plant Fiber Polymer Composites Performances
5.1. Physical Properties
5.2. Mechanical Properties
5.3. Thermal Properties
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, L.C.; Sapuan, S.M.; Hassan, M.R.; Sheltami, R.M. 2-Natural fiber reinforced vinyl polymer composites. In Natural Fiber Reinforced Vinyl Ester and Vinyl Polymer Composites; Sapuan, S.M., Ismail, H., Zainudin, E.S., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 27–70. [Google Scholar]
- Li, M.; Pu, Y.; Thomas, V.M.; Yoo, C.G.; Ozcan, S.; Deng, Y.; Nelson, K.; Ragauskas, A.J. Recent advancements of plant-based natural fiber–reinforced composites and their applications. Compos. Part B Eng. 2020, 200, 108254. [Google Scholar] [CrossRef]
- AZoM. Composite Matrix Materials. Available online: https://www.azom.com/article.aspx?ArticleID=9814 (accessed on 20 November 2020).
- Lee, C.H.; Sapuan, S.M.; Hassan, M.R. Mechanical and Thermal Properties of Kenaf Fiber Reinforced Polypropylene/Magnesium Hydroxide Composites. J. Eng. Fibers Fabr. 2017, 12, 155892501701200206. [Google Scholar] [CrossRef] [Green Version]
- Faria, L.U.S.; Pacheco, B.J.S.; Oliveira, G.C.; Silva, J.L. Production of cellulose nanocrystals from pineapple crown fibers through alkaline pretreatment and acid hydrolysis under different conditions. J. Mater. Res. Technol. 2020, 9, 12346–12353. [Google Scholar] [CrossRef]
- Sakuri, S.; Surojo, E.; Ariawan, D. Thermogravimetry and Interfacial Characterization of Alkaline Treated Cantala fiber/Microcrystalline Cellulose-Composite. Procedia Struct. Integr. 2020, 27, 85–92. [Google Scholar] [CrossRef]
- Ismail, S.H.; Abu Bakar, A. The Effect of Compatibilizer and Coupling Agent on the Properties of Paper Sludge Filled Polypropylene (PP)/Ethylene Propylene Diene Terpolymer (EPDM) Composites. Polym. Plast. Technol. Eng. 2005, 44, 863–879. [Google Scholar] [CrossRef]
- Khalid, M.; Salmiaton, A.; Chuah, T.G.; Ratnam, C.T.; Choong, S.Y.T. Effect of MAPP and TMPTA as compatibilizer on the mechanical properties of cellulose and oil palm fiber empty fruit bunch–polypropylene biocomposites. Compos. Interfaces 2008, 15, 251–262. [Google Scholar] [CrossRef]
- Hamour, N.; Boukerrou, A.; Djidjelli, H.; Maigret, J.-E.; Beaugrand, J. Effects of MAPP Compatibilization and Acetylation Treatment Followed by Hydrothermal Aging on Polypropylene Alfa Fiber Composites. Int. J. Polym. Sci. 2015, 2015, 451691. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Lee, B.-H.; Choi, S.-W.; Kim, S.; Kim, H.-J. The effect of types of maleic anhydride-grafted polypropylene (MAPP) on the interfacial adhesion properties of bio-flour-filled polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1473–1482. [Google Scholar] [CrossRef]
- Ugochukwu, S.; Ridzuan, M.J.M.; Majid, M.S.A.; Cheng, E.M.; Firdaus, A.Z.A.; Marsi, N. Influence of distilled water and alkaline solution on the scratch resistance properties of Napier fiber filled epoxy (NFFE) composites. J. Mater. Res. Technol. 2020, 9, 14412–14424. [Google Scholar] [CrossRef]
- Cho, D.; Kim, H.J.; Drzal, L.T. Surface Treatment and Characterization of Natural Fibers: Effects on the Properties of Biocomposites. In Polymer Composites; Thomas, S., Joseph, K., Malhotra, S.K., Goda, K., Sreekala, M.S., Eds.; Wiley-VCH: Weinheim, Germany, 2013; Volume 3, pp. 133–177. [Google Scholar]
- Kumar, D.; Raj, G.; Shivaani, G.; Sreehari, V.M. Structural analysis of aircraft wings made of natural fiber reinforced composites. Int. J. Mech. Eng. Technol. 2018, 9, 1262–1268. [Google Scholar]
- Jesuarockiam, N.; Jawaid, M.; Saba, N. Sustainable Bio Composites for Aircraft Components. In Sustainable Composies for Aerospace Applications; Jawaid, M., Thariq, M., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 109–123. [Google Scholar]
- Cicala, G.; Cristaldi, G.; Recca, G.; Latteri, A. Composites Based on Natural Fiber Fabrics. In Woven Fabric Engineering; Dubrovski P., D., Ed.; IntechOpen: London, UK, 2010; pp. 317–342. [Google Scholar]
- Shetty, R.; Pai, R.; Barboza, A.B.V.; Gandhi, V.P. Processing, mechanical charaterization and its tribological study of discontinously reinforced Caryota Urens Fiber Polyester composites. ARPN J. Eng. Appl. Sci. 2018, 13, 3920–3928. [Google Scholar]
- Sathishkumar, T.P.; Navaneethakrishnan, P.; Subramaniam, S.; Rajasekar, R.; Rajini, N. Characterization of natural fiber and composites-A review. J. Reinf. Plast. Compos. 2013, 32, 1457–1476. [Google Scholar] [CrossRef]
- Lee, C.H.; Salit, M.S.; Hassan, M.R. A Review of the Flammability Factors of Kenaf and Allied Fiber Reinforced Polymer Composites. Adv. Mater. Sci. Eng. 2014, 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Samadi, M.; Zainal Abidin, Z.; Yoshida, H.; Yunus, R.; Awang Biak, D.R.; Lee, C.H.; Lok, E.H. Subcritical water extraction of essential oil from Aquilaria malaccensis leaves. Sep. Sci. Technol. 2019, 55, 2779–2798. [Google Scholar] [CrossRef]
- Dashtizadeh, Z.; Khalina, A.; Cardona, F.; Lee, C.H. Mechanical Characteristics of Green Composites of Short Kenaf Bast Fiber Reinforced in Cardanol. Adv. Mater. Sci. Eng. 2019, 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Kabir, M.M.; Wang, H.; Aravinthan, T.; Cardona, F.; Lau, K.-T. Effects of natural fiber surface on composite properties: A review. In Proceedings of the 1st International Postgraduate Conference on Engineering, Designing and Developing the Built Environment for Sustainable Wellbeing, Brisbane, Australia, 27–29 April 2011. [Google Scholar]
- Amiandamhen, S.O.; Meincken, M.; Tyhoda, L. Natural Fiber Modification and Its Influence on Fiber-matrix Interfacial Properties in Biocomposite Materials. Fibers Polym. 2020, 21, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Gholampour, A.; Ozbakkaloglu, T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2020, 55, 829–892. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef] [Green Version]
- Ticoalu, A.; Aravinthan, T.; Cardona, F. A study into the characteristics of gomuti (Arenga pinnata) fiber for usage as natural fiber composites. J. Reinf. Plast. Compos. 2014, 33, 179–192. [Google Scholar]
- Liu, K.; Takagi, H.; Osugi, R.; Yang, Z. Effect of physicochemical structure of natural fiber on transverse thermal conductivity of unidirectional abaca/bamboo fiber composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1234–1241. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Chen, L. Interface and bonding mechanisms of plant fiber composites: An overview. Compos. Part B Eng. 2016, 101, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.Q.N.; Fuentes, C.A.; Dupont-Gillain, C.; Van Vuure, A.W.; Verpoest, I. Wetting analysis and surface characterisation of coir fibers used as reinforcement for composites. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 251–260. [Google Scholar] [CrossRef]
- Tran, L.Q.N.; Fuentes, C.; Dupont, C.; Vuure, A.W.; Verpoest, I. Understanding the interfacial compatibility and adhesion of natural coir fiber thermoplastic composites. Compos. Sci. Technol. 2013, 80, 23–30. [Google Scholar] [CrossRef]
- Tran, L.Q.N.; Yuan, X.; Bhattacharyya, D.; Fuentes, C.; Vuure, A.W.; Verpoest, I. Fiber-matrix interfacial adhesion in natural fiber composites. Int. J. Mod. Phys. B 2015, 29, 1540018. [Google Scholar] [CrossRef]
- Fuentes, C.; Tran, L.Q.N.; Hellemont, M.; Janssens, V.; Dupont, C.; Vuure, A.W.; Verpoest, I. Effect of physical adhesion on mechanical behaviour of bamboo fiber reinforced thermoplastic composites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 7–15. [Google Scholar] [CrossRef]
- Effah, B.; Van Reenen, A.; Meincken, M. Characterisation of the Interfacial Adhesion of the Different Components in Wood–Plastic Composites with AFM. Springer Sci. Rev. 2015, 3, 97–111. [Google Scholar] [CrossRef]
- Heath, C.; Bond, I.; Potter, K. Integrating electrostatic adhesion to composite structures. Proceedings of SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, San Diego, CA, USA, 8–12 March 2005; pp. 1–11. [Google Scholar]
- Heath, C.; Bond, I.; Potter, K. Variable Stiffness Sandwich Panels Using Electrostatic Interlocking Core. In Proceedings of the. SPIE 9799, Active and Passive Smart Structures and Integrated Systems, Las Vegas, NV, USA, 20–24 March 2016; pp. 1–10. [Google Scholar]
- Heath, C.; Bond, I.; Potter, K. Electrostatic adhesion for added functionality of composite structures. Smart Mater. Struct. 2016, 25, 025016. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.M.; Shyng, Y.T. Surface modification of PBO fiber by electrostatic discharge for composites. J. Achiev. Mater. Manuf. Eng. 2006, 28, 169–172. [Google Scholar]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Khalit, M.; Anuar, H.; Shaffiar, N.; Yaacob, I.; Sapuan, S. Matrix Cracking in Reinforced Polymer Nanocomposites: A Review. J. Adv. Sci. 2015, 11, 13–36. [Google Scholar]
- Balla, V.K.; Kate, K.H.; Satyavolu, J.; Singh, P.; Tadimeti, J.G.D. Additive manufacturing of natural fiber reinforced polymer composites: Processing and prospects. Compos. Part B Eng. 2019, 174, 106956. [Google Scholar] [CrossRef]
- Oushabi, A.; Oudrhiri Hassani, F.; Abboud, Y.; Sair, S.; Tanane, O.; El Bouari, A. Improvement of the interface bonding between date palm fibers and polymeric matrices using alkali-silane treatments. Int. J. Ind. Chem. 2018, 9, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Putra, A.E.E.; Renreng, I.; Arsyad, H.; Bakri, B. Investigating the effects of liquid-plasma treatment on tensile strength of coir fibers and interfacial fiber-matrix adhesion of composites. Compos. Part B Eng. 2020, 183, 107722. [Google Scholar] [CrossRef]
- Sethi, S.; Ray, B.C. Environmental effects on fiber reinforced polymeric composites: Evolving reasons and remarks on interfacial strength and stability. Adv. Colloid Interface Sci. 2015, 217, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Malhotra, K.; Thomas, S.; Joseph, K.; Koichi Goda, K.; Sreekala, M. State of the art, new challenges, andcopportunities. In Polymer Composites, Macro- and Microcomposites; Sabu, T., Kuruvilla, J., Malhotra, S.K., Koichi, G., Sreekala, M.S., Eds.; Wiley-VCH: Weinhein, Germany, 2012; Volume 1. [Google Scholar]
- Hamad, S.F.; Stehling, N.; Hayes, S.A.; Foreman, J.P.; Rodenburg, C. Exploiting Plasma Exposed, Natural Surface Nanostructures in Ramie Fibers for Polymer Composite Applications. Materials 2019, 12, 1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, E.; Panigrahi, S. Effect of Plasma Treatment on Structure, Wettability of Jute Fiber and Flexural Strength of its Composite. J. Compos. Mater. 2009, 43, 1791–1802. [Google Scholar] [CrossRef]
- Imran, M.; Artian, S.; Silvy, D.B. Wettability Properties of Coconut Fiber on Resin. Int. J. Sci. Res. 2018, 7, 1432–1434. [Google Scholar]
- Samajpati, S.; Sengupta, S. Wetting characteristics of long vegetable fibers. Indian J. Fiber Text. Res. 2006, 31, 262–266. [Google Scholar]
- Matthias, W.; Mariagrazia, T. Surface Wettability: To Alter Surface Wettability. Available online: https://www.igvp.uni-stuttgart.de/en/research/plasma-technology/processes/surface-wettability/ (accessed on 5 November 2020).
- Gamelas, J.A.F. The surface properties of cellulose and lignocellulosic materials assessed by inverse gas chromatography: A review. Cellulose 2013, 20, 2675–2693. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, N.; Gouveia, C.; Moraes, A.G.O.; Amico, S.C. Natural fibers characterization by inverse gas chromatography. Carbohydr. Polym. 2011, 84, 110–117. [Google Scholar] [CrossRef]
- Cordeiro, N.; Ornelas, M.; Ashori, A.; Sheshmani, S.; Norouzi, H. Investigation on the surface properties of chemically modified natural fibers using inverse gas chromatography. Carbohydr. Polym. 2012, 87, 2367–2375. [Google Scholar] [CrossRef]
- Matuana, L.; Balatinecz, J.; Park, C.; Woodhams, R.T. Surface characteristics of chemically modified newsprint fibers determined by inverse gas chromatography. Wood Fiber Sci. 2007, 31, 116–127. [Google Scholar]
- Cordeiro, N.; Gouveia, C.; John, M.J. Investigation of surface properties of physico-chemically modified natural fibers using inverse gas chromatography. Ind. Crop. Prod. 2011, 33, 108–115. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Germinario, L.; Winter, W. Isolation, preparation and characterization of cellulose microfibers obtained from bagasse. Carbohydr. Polym. 2008, 73, 371–377. [Google Scholar] [CrossRef]
- Hashim, M.Y.; Roslan, M.N.; Mohd Amin, A.; Zaidi, A.M.A.; Ariffin, S. Mercerization treatment parameter effect on natural fiber reinforced polymer matrix composite: A brief review. World Acad. Sci. Eng. Technol. 2012, 68, 1638–1644. [Google Scholar]
- Santos, J.; Siqueira, R.; Vieira, L.; Freire, R.; Mano, V.; Panzera, T. Effects of sodium carbonate on the performance of epoxy and polyester coir-reinforced composites. Polym. Test. 2018, 67, 533–544. [Google Scholar] [CrossRef]
- Akhtar, M.; Bakar, A.; Md Radzi, M.K.F.; Ismail, N.; Raza, M.; Muhamad, N.; Khan, M. Influence of alkaline treatment and fiber loading on the physical and mechanical properties of kenaf/polypropylene composites for variety of applications. Prog. Nat. Sci. Mater. Int. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Rajini, N. Mechanical properties of banana/kenaf fiber-reinforced hybrid polyester composites: Effect of woven fabric and random orientation. Mater. Des. 2014, 66(A), 246–257. [Google Scholar]
- Oml, A.; Reid, R.; Paskaramoorthy, R. The effects of alkali-silane treatment on the tensile and flexural properties of short fiber non-woven kenaf reinforced polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1431–1440. [Google Scholar]
- Mokhothu, T.H.; John, M.J. Bio-based coatings for reducing water sorption in natural fiber reinforced composites. Sci. Rep. 2017, 7, 13335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurul Atiqah, M.S.; Gopakumar, D.A.; Owolabi, F.A.T.; Pottathara, Y.B.; Rizal, S.; Sri Aprilia, N.A.; Hermawan, D.; Paridah, M.T.T.; Thomas, S.; Abdul Khalil, H.P.S. Extraction of Cellulose Nanofibers via Eco-friendly Supercritical Carbon Dioxide Treatment Followed by Mild Acid Hydrolysis and the Fabrication of Cellulose Nanopapers. Polymers 2019, 11, 1813. [Google Scholar]
- Huang, P.; Zhao, H.; Kuga, S.; Wu, M.; Huang, Y. A versatile method to produce functionalized cellulose nanofiber and its application. Nanoscale 2016, 8, 3753–3759. [Google Scholar] [CrossRef] [PubMed]
- Dima, Ş.-O.; Panaitescu, D.; Orbán, C.; Ghiurea, M.; Doncea, S.; Fierascu, R.; Nistor, C.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuruddin, M.; Hosur, M.; Uddin, M.J.; Baah, D.; Jeelani, S. A novel approach for extracting cellulose nanofibers from lignocellulosic biomass by ball milling combined with chemical treatment. J. Appl. Polym. Sci. 2016, 133, 42990. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.-L.; Restrepo Osorio, A.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana farming residues: Isolation and characterization. Cellulose 2007, 14, 585–592. [Google Scholar] [CrossRef]
- Zhanying, S. Progress in the research and applications of natural fiber-reinforced polymer matrix composites. Sci. Eng. Compos. Mater. 2018, 25, 835–846. [Google Scholar]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fiber composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Mecánica, I.; Jaramillo, N.; Hoyos, D.; Santa, J. Composites with pineapple-leaf fibers manufactured by layered compression molding moldeo por compresión por capas. Ing. Y Compet. 2016, 18, 151–162. [Google Scholar]
- Kafi, A.A.; Magniez, K.; Fox, B.L. A surface-property relationship of atmospheric plasma treated jute composites. Compos. Sci. Technol. 2011, 71, 1692–1698. [Google Scholar] [CrossRef]
- Mat Taib, R.; Ariawan, D.; Ishak, Z. Surface Characterization of Alkali Treated Kenaf Fibers by XPS and AFM. Key Eng. Mater. 2016, 694, 29–33. [Google Scholar]
- Parvej, M.S.; Wang, X.; Jiang, L. AFM Based Nanomechanical Characterization of Cellulose Nanofibril. J. Compos. Mater. 2020, 54, 4487–4493. [Google Scholar] [CrossRef]
- Melelli, A.; Arnould, O.; Beaugrand, J.; Bourmaud, A. The Middle Lamella of Plant Fibers Used as Composite Reinforcement: Investigation by Atomic Force Microscopy. Molecules 2020, 25, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.Y.; Ross, R.J. Relationship between radial compressive modulus of elasticity and shear modulus of wood. Wood Fiber Sci. 2005, 37, 201–206. [Google Scholar]
- Ching Hao, L.; Abdan, K.; Lee, S.H.; Liu, M. A Comprehensive Review on Bast Fiber Retting Process for Optimal Performance in Fiber-Reinforced Polymer Composites. Adv. Mater. Sci. Eng. 2020, 2020, 1–27. [Google Scholar]
- Komuraiah, A.; Kumar, N.S.; Prasad, B.D. Chemical Composition of Natural Fibers and its Influence on their Mechanical Properties. Mech. Compos. Mater. 2014, 50, 359–376. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A. Chemical Properties of Natural Fiber Composites and Mechanisms of Chemical Modifications. Asian J. Chem. 2012, 24, 1831–1836. [Google Scholar]
- Tserki, V.; Zafeiropoulos, N.E.; Simon, F.; Panayiotou, C. A study of the effect of acetylation and propionylation surface treatments on natural fibers. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1110–1118. [Google Scholar] [CrossRef]
- Manimaran, P.; Senthamaraikannan, P.; Sanjay, M.R.; Marichelvam, M.K.; Jawaid, M. Study on characterization of Furcraea foetida new natural fiber as composite reinforcement for lightweight applications. Carbohydr. Polym. 2018, 181, 650–658. [Google Scholar] [CrossRef]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Morphology, Structure, and Mechanical Properties of Natural Cellulose Fiber from Mendong Grass (Fimbristylis globulosa). J. Nat. Fibers 2014, 11, 333–351. [Google Scholar] [CrossRef]
- Singh, H.; Chatterjee, A.; Kumar, S. Tensile strength and thermal behavior of jute fiber reinforced polypropylene laminate composite. Compos. Commun. 2020, 22, 100483. [Google Scholar]
- Mizi, F. Fourier Transform Infrared Spectroscopy for Natural Fibers. In Fourier Transform-Materials Analysis; Salih, S.M., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Shao, X.; He, L.; Ma, L. Water Absorption and FTIR Analysis of Three Type Natural Fiber Reinforced Composites. In Proceedings of the 2015 2nd International Forum on Electrical Engineering and Automation (IFEEA 2015), GuangZhou, China, 26–27 December 2015. [Google Scholar]
- Schwarzova, I.; Stevulova, N.; Singovszka, E.; Terpakova, E. Surface Treated Natural Fibers as Filler in Biocomposites. Iop Conf. Ser. Mater. Sci. Eng. 2015, 96, 012028. [Google Scholar] [CrossRef] [Green Version]
- Corgié, S.C.; Smith, H.M.; Walker, L.P. Enzymatic transformations of cellulose assessed by quantitative high-throughput fourier transform infrared spectroscopy (QHT-FTIR). Biotechnol. Bioeng. 2011, 108, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Madhu, P.; Sanjay, M.R.; Jawaid, M.; Siengchin, S.; Khan, A.; Pruncu, C.I. A new study on effect of various chemical treatments on Agave Americana fiber for composite reinforcement: Physico-chemical, thermal, mechanical and morphological properties. Polym. Test. 2020, 85, 106437. [Google Scholar] [CrossRef]
- Ganapathy, T.; Sathiskumar, R.; Senthamaraikannan, P.; Saravanakumar, S.S.; Khan, A. Characterization of raw and alkali treated new natural cellulosic fibers extracted from the aerial roots of banyan tree. Int. J. Biol. Macromol. 2019, 138, 573–581. [Google Scholar] [CrossRef]
- Vijay, R.; Vinod, A.; Lenin Singaravelu, D.; Sanjay, M.R.; Siengchin, S. Characterization of chemical treated and untreated natural fibers from Pennisetum orientale grass- A potential reinforcement for lightweight polymeric applications. Int. J. Lightweight Mater. Manuf. 2021, 4, 43–49. [Google Scholar] [CrossRef]
- Oladele, I.; Michael, O.J.; Olusegun, S. Influence of Chemical Treatment on the Constituents and Tensile Properties of Selected Agro-Fibers. West Indian J. Eng. 2016, 38, 569–582. [Google Scholar]
- Li, Z.; Yu, C. Effect of peroxide and softness modification on properties of ramie fiber. Fibers Polym. 2014, 15, 2105–2111. [Google Scholar] [CrossRef]
- Amiri, A.; Ulven, C.A.; Huo, S. Effect of Chemical Treatment of Flax Fiber and Resin Manipulation on Service Life of Their Composites Using Time-Temperature Superposition. Polymers 2015, 7, 1965–1978. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, Z.; Ahmad, M.; Aziz, A.A.; Ramli, R.; Hassan, K.; Alias, A.H. Properties of chemically treated oil palm Empty Fruit Bunch (EFB) fibers. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 57, 57–68. [Google Scholar]
- Mosiewicki, M.A.; Marcovich, N.E.; Aranguren, M.I. 4-Characterization of fiber surface treatments in natural fiber composites by infrared and Raman spectroscopy. In Interface Engineering of Natural Fiber Composites for Maximum Performance; Zafeiropoulos, N.E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 117–145. [Google Scholar]
- Zhong, W.; Pan, N. A Computer Simulation of Single Fiber Pull Out Process in a Composite. J. Compos. Mater. 2003, 37, 1951–1969. [Google Scholar] [CrossRef]
- Notta-Cuvier, D.; Lauro, F.; Bennani, B. Modelling of progressive fiber/matrix debonding in short-fiber reinforced composites up to failure. Int. J. Solids Struct. 2015, 66, 140–150. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Salit, M.S. Effects of Surface Treatments on Tensile, Thermal and Fiber-matrix Bond Strength of Coir and Pineapple Leaf Fibers with Poly Lactic Acid. J. Bionic Eng. 2018, 15, 1035–1046. [Google Scholar] [CrossRef]
- Viel, Q.; Esposito, A.; Saiter, J.-M.; Santulli, C.; Turner, J.A. Interfacial Characterization by Pull-Out Test of Bamboo Fibers Embedded in Poly(Lactic Acid). Fibers 2018, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-S.; Koo, W.-M.; Kim, H.-D. Preparation and Properties of New Regenerated Cellulose Fibers. Text. Res. J. 2003, 73, 998–1004. [Google Scholar] [CrossRef]
- Obi Reddy, K.; Uma Maheswari, C.; Shukla, M.; Song, J.I.; Varada Rajulu, A. Tensile and structural characterization of alkali treated Borassus fruit fine fibers. Compos. Part B Eng. 2013, 44, 433–438. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Nicoletti, F.; Vitale, G.; Prestipino, M.; Valenza, A. A new eco-friendly chemical treatment of natural fibers: Effect of sodium bicarbonate on properties of sisal fiber and its epoxy composites. Compos. Part B Eng. 2016, 85, 150–160. [Google Scholar] [CrossRef]
- Mazzanti, V.; Salzano de Luna, M.; Pariante, R.; Mollica, F.; Filippone, G. Natural fiber-induced degradation in PLA-hemp biocomposites in the molten state. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105990. [Google Scholar] [CrossRef]
- Fiore, V.; Sanfilippo, C.; Calabrese, L. Influence of sodium bicarbonate treatment on the aging resistance of natural fiber reinforced polymer composites under marine environment. Polym. Test. 2019, 80, 106100. [Google Scholar] [CrossRef]
- Gassan, J.; Bledzki, A.K. Possibilities for improving the mechanical properties of jute/epoxy composites by alkali treatment of fibers. Compos. Sci. Technol. 1999, 59, 1303–1309. [Google Scholar] [CrossRef]
- Aziz, S.H.; Ansell, M.P. The effect of alkalization and fiber alignment on the mechanical and thermal properties of kenaf and hemp bast fiber composites: Part 1–polyester resin matrix. Compos. Sci. Technol. 2004, 64, 1219–1230. [Google Scholar] [CrossRef]
- De Farias, J.G.G.; Cavalcante, R.C.; Canabarro, B.R.; Viana, H.M.; Scholz, S.; Simão, R.A. Surface lignin removal on coir fibers by plasma treatment for improved adhesion in thermoplastic starch composites. Carbohydr. Polym. 2017, 165, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Goriparthi, B.K.; Suman, K.N.S.; Mohan Rao, N. Effect of fiber surface treatments on mechanical and abrasive wear performance of polylactide/jute composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1800–1808. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Characterization of cellulose fibers in Thespesia populnea barks: Influence of alkali treatment. Carbohydr. Polym. 2019, 217, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Mohan Bhasney, S.; Mondal, K.; Kumar, A.; Katiyar, V. Effect of microcrystalline cellulose [MCC] fibers on the morphological and crystalline behaviour of high density polyethylene [HDPE]/polylactic acid [PLA] blends. Compos. Sci. Technol. 2020, 187, 107941. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Y.; Qiu, J.; Fei, M.; Qiu, R.; Liu, W. Interfacial compatibilization via in-situ polymerization of epoxidized soybean oil for bamboo fibers reinforced poly(lactic acid) biocomposites. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106066. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Liu, Y.; Yang, G.C.; Zeng, H.M. The effect of fiber treatment on the mechanical properties of unidirectional sisal-reinforced epoxy composites. Compos. Sci. Technol. 2001, 61, 1437–1447. [Google Scholar] [CrossRef]
- Woigk, W.; Fuentes, C.A.; Rion, J.; Hegemann, D.; van Vuure, A.W.; Dransfeld, C.; Masania, K. Interface properties and their effect on the mechanical performance of flax fiber thermoplastic composites. Compos. Part A Appl. Sci. Manuf. 2019, 122, 8–17. [Google Scholar] [CrossRef]
- Rahman, M.R.; Hamdan, S.; Jayamani, E.; Kakar, A.; Bakri, M.K.B.; Yusof, F.A.B.M. Tert-butyl catechol/alkaline-treated kenaf/jute polyethylene hybrid composites: Impact on physico-mechanical, thermal and morphological properties. Polym. Bull. 2019, 76, 763–784. [Google Scholar] [CrossRef]
- Narayanasamy, P.; Balasundar, P.; Senthil, S.; Sanjay, M.R.; Siengchin, S.; Khan, A.; Asiri, A.M. Characterization of a novel natural cellulosic fiber from Calotropis gigantea fruit bunch for ecofriendly polymer composites. Int. J. Biol. Macromol. 2020, 150, 793–801. [Google Scholar] [CrossRef]
- Chung, T.-J.; Park, J.-W.; Lee, H.-J.; Kwon, H.-J.; Kim, H.-J.; Lee, Y.-K.; Tze, W. The Improvement of Mechanical Properties, Thermal Stability, and Water Absorption Resistance of an Eco-Friendly PLA/Kenaf Biocomposite Using Acetylation. Appl. Sci. 2018, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Sumesh, K.R.; Kanthavel, K.; Kavimani, V. Peanut oil cake-derived cellulose fiber: Extraction, application of mechanical and thermal properties in pineapple/flax natural fiber composites. Int. J. Biol. Macromol. 2020, 150, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.H.; Ansell, M.P. The effect of alkalization and fiber alignment on the mechanical and thermal properties of kenaf and hemp bast fiber composites: Part 2–cashew nut shell liquid matrix. Compos. Sci. Technol. 2004, 64, 1231–1238. [Google Scholar] [CrossRef]
- Nielsen, L.E.; Landel, R.F. Mechanical Properties of Polymers and Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1974. [Google Scholar]
- Wu, Y.; Fei, M.; Chen, T.; Qiu, R.; Liu, W. Biocomposites from bamboo fibers and palm oil-based thermosets: Effects of natural phenolic cross-linkers. Compos. Commun. 2020, 22, 100489. [Google Scholar] [CrossRef]
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Novel biodegradable polymer films based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life 2020, 25, 100538. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S.; Tábi, T.; Kovács, J. Comparison of injection moulded, natural fiber-reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mater. 2012, 25, 927–948. [Google Scholar] [CrossRef]
- Tripathy, S.; Jali, P.; Parida, C.; Pradhan, C. Study on biodegradability and thermal behaviour of composites using poly lactic acid and gamma-irradiated fibers of Luffa cylindrica. Chemosphere 2020, 261, 127684. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Fierascu, R.C.; Gabor, A.R.; Nicolae, C.A. Effect of hemp fiber length on the mechanical and thermal properties of polypropylene/SEBS/hemp fiber composites. J. Mater. Res. Technol. 2020, 9, 10768–10781. [Google Scholar] [CrossRef]
- Asim, M.; Paridah, M.T.; Saba, N.; Jawaid, M.; Alothman, O.Y.; Nasir, M.; Almutairi, Z. Thermal, physical properties and flammability of silane treated kenaf/pineapple leaf fibers phenolic hybrid composites. Compos. Struct. 2018, 202, 1330–1338. [Google Scholar] [CrossRef]
- Garcia Filho, F.D.C.; Da Luz, F.S.; Oliveira, M.S.; Pereira, A.C.; Costa, U.O.; Monteiro, S.N. Thermal behavior of graphene oxide-coated piassava fiber and their epoxy composites. J. Mater. Res. Technol. 2020, 9, 5343–5351. [Google Scholar] [CrossRef]








| Fibers | Cellulose (wt%) | Hemicellulose (wt%) | Lignin (wt%) | Waxes (wt%) |
|---|---|---|---|---|
| Bagasse | 55.2 | 16.8 | 25.3 | - |
| Bamboo | 26–43 | 30 | 21–31 | - |
| Flax | 71 | 18.6–20.6 | 2.2 | 1.5 |
| Kenaf | 72 | 20.3 | 9 | - |
| Jute | 61–71 | 14–20 | 12–13 | 0.5 |
| Hemp | 68 | 15 | 10 | 0.8 |
| Ramie | 68.6–76.2 | 13–16 | 0.6–0.7 | 0.3 |
| Abaca | 56–63 | 20–25 | 7–9 | 3 |
| Sisal | 65 | 12 | 9.9 | 2 |
| Coir | 32–43 | 0.15–0.25 | 40–45 | - |
| Oil palm | 65 | - | 29 | - |
| Pineapple | 81 | - | 12.7 | - |
| Curaua | 73.6 | 9.9 | 7.5 | - |
| Wheat straw | 38–45 | 15–31 | 12–20 | - |
| Rice husk | 35–45 | 19–25 | 20 | - |
| Rice straw | 41–57 | 33 | 8–19 | 8–38 |
| Steps | Fiber Pull-Out Stages |
|---|---|
| 1 | Initiation of interfacial microfailure at fiber tips due to tensile stress concentration in matrix around fiber tips: from about 50% of ultimate load |
| 2 | Separation at the interface, formation of a microvoid. |
| 3 | Propagation of interfacial microfailures along fiber sides due to critical shear stress concentration: from about 75% of ultimate load; a fringe pattern of shear mode and microcracks are observed in the matrix along fiber sides. |
| 4 | Occurrence of plastic deformation bands in the matrix due to stress concentration caused by the reduction of fiber load bearing capability; crack opening and slow crack propagation through plastic deformation bands (ductile crack propagation). |
| 5 | Brittle crack propagation: when crack size reaches a critical value (about 1 mm), they propagate along fiber sides and through the matrix, which leads to composite failure. |
| Possible Pull-Out Conditions | |
|---|---|
| (a) Low Interfacial Bonding Strength |  |
| (b) Medium Interfacial Bonding Strength |  |
| (c) High Interfacial Bonding Strength |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.H.; Khalina, A.; Lee, S.H. Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review. Polymers 2021, 13, 438. https://doi.org/10.3390/polym13030438
Lee CH, Khalina A, Lee SH. Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review. Polymers. 2021; 13(3):438. https://doi.org/10.3390/polym13030438
Chicago/Turabian StyleLee, Ching Hao, Abdan Khalina, and Seng Hua Lee. 2021. "Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review" Polymers 13, no. 3: 438. https://doi.org/10.3390/polym13030438