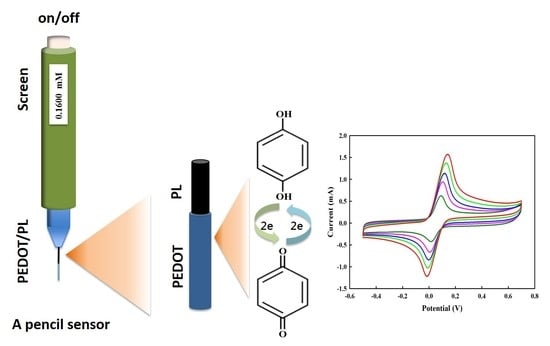
Fabrication of an Extremely Cheap Poly(3,4-ethylenedioxythiophene) Modified Pencil Lead Electrode for Effective Hydroquinone Sensing
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Construction of a PEDOT/PL Electrode
2.3. Characterizations of PEDOT/PL
2.4. Measurement of Hydroquinone by the Constructed PEDOT/PL Electrode
3. Experimental Results
3.1. Biosensor Fabrication and Characterizations
3.2. Characterization of PEDOT/PL by FTIR
3.3. Detection of Hydroquinone with a PEDOT/PL Electrode
3.4. The effect of pH on the Detection
3.5. The Effect of Synthesis Cycle for PEDOT Film on the Performance
3.6. Stability of PEDOT/PL for Multiple Measurements
3.7. Potential Interference on the Measurement
3.8. The Linear Sweep Voltammetry (LSV) Measurement of HQ with the PEDOT/PL Electrode
3.9. The Practical Applications and Future Research Perspectives of Conductive PEDOT in Biosensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorensen, M.; Skov, H.; Autrup, H.; Hertel, O.; Loft, S. Urban benzene exposure and oxidative DNA damage: Influence of genetic polymorphisms in metabolism genes. Sci. Total Environ. 2003, 309, 69–80. [Google Scholar] [CrossRef]
- Costa-Amaral, I.C.; Carvalho, L.V.B.; Santos, M.V.C.; Valente, D.; Pereira, A.C.; Figueiredo, V.O.; de Souza, J.M.; Castro, V.S.; Trancoso, M.D.; Fonseca, A.S.A.; et al. Environmental Assessment and Evaluation of Oxidative Stress and Genotoxicity Biomarkers Related to Chronic Occupational Exposure to Benzene. Int. J. Environ. Res. Pub. Health 2019, 16, 2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, N.; Naddafi, K.; Nabizadeh, R.; Yaghmaeian, K.; Assanvand, M.S.; Maroufizadeh, S.; Hoseini, M.; Adabi, S.; Yunesian, M. Urinary benzene as a biomarker of environmental exposure to benzene in males in the general population. Acta Med. Mediterr. 2016, 32, 1471–1475. [Google Scholar]
- Angelini, S.; Maffei, F.; Bermejo, J.L.; Ravegnini, G.; L’Insalata, D.; Cantelli-Forti, G.; Violante, F.S.; Hrelia, P. Environmental exposure to benzene, micronucleus formation and polymorphisms in DNA-repair genes: A pilot study. Mutat. Res. Gen. Tox. Environ. Mutagenesis 2012, 743, 99–104. [Google Scholar] [CrossRef]
- Kok, P.W.; Ong, H.Y.; Wong, M.K.; Au, W.K.; Tan, K.T.; Phoon, W.H.; Ong, C.N. Environmental and biological assessment of exposure to benzene in petroleum workers. Environ. Monit. Assess. 1997, 44, 425–431. [Google Scholar] [CrossRef]
- Wallace, L. Environmental exposure to benzene: An update. Environ. Health Persp. 1996, 104, 1129–1136. [Google Scholar]
- Deisinger, P.J.; Hill, T.S.; English, J.C. Human exposure to naturally occurring hydroquinone. J. Toxicol. Environ. Health 1996, 47, 31–46. [Google Scholar] [CrossRef]
- Irons, R.D. Quinones as toxic metabolites of benzene. J. Toxicol. Environ. Health 1985, 16, 673–678. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, H.M.; Shi, G.Y.; Ong, G.N. Benzene metabolites enhance reactive oxygen species generation in hl60 human leukemia cells. Hum. Exp. Toxicol. 1996, 15, 422–427. [Google Scholar] [CrossRef]
- Horita, M.; Wang, D.H.; Tsutsui, K.; Sano, K.; Masuoka, N.; Kira, S. Involvement of oxidative stress in hydroquinone-induced cytotoxicity in catalase-deficient escherichia coli mutants. Free Radic. Res. 2005, 39, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Sze, C.C.; Shi, C.Y.; Ong, C.N. Cytotoxicity and DNA strand breaks induced by benzene and its metabolites in chinese hamster ovary cells. J. Appl. Toxicol. 1996, 16, 259–264. [Google Scholar] [CrossRef]
- Matsumoto, M.; Masumori, S.; Hirata-Koizumi, M.; Ono, A.; Honma, M.; Yokoyama, K.; Hirose, A. Evaluation of in vivo mutagenicity of hydroquinone in muta (tm) mice. Mutat. Res. Gen. Tox. Environ. Mutagenesis 2014, 775, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Liang, H.; Hu, G.H.; Yang, H.; Zhou, K.; Xu, L.M.; Liu, J.X.; Lai, B.; Song, L.; Luo, H.; et al. Differently expressed long noncoding rnas and mrnas in tk6 cells exposed to low dose hydroquinone. Oncotarget 2017, 8, 95554–95567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahammad, A.J.S.; Rahman, M.M.; Xu, G.R.; Kim, S.; Lee, J.J. Highly sensitive and simultaneous determination of hydroquinone and catechol at poly(thionine) modified glassy carbon electrode. Electrochim. Acta 2011, 56, 5266–5271. [Google Scholar] [CrossRef]
- Kerzic, P.J.; Liu, W.S.; Pan, M.T.; Fu, H.; Zhou, Y.; Schnatter, A.R.; Irons, R.D. Analysis of hydroquinone and catechol in peripheral blood of benzene-exposed workers. Chem. Biol. Interact. 2010, 184, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Pistonesi, M.F.; Di Nezio, M.S.; Centurion, M.; Palomeque, M.E.; Lista, A.G.; Band, B.S.F. Determination of phenol, resorcinol and hydroquinone in air samples by synchronous fluorescence using partial least-squares (pls). Talanta 2006, 69, 1265–1268. [Google Scholar] [CrossRef]
- Marrubini, G.; Calleri, E.; Coccini, T.; Castoldi, A.F.; Manzo, L. Direct analysis of phenol, catechol and hydroquinone in human urine by coupled-column hplc with fluorimetric detection. Chromatographia 2005, 62, 25–31. [Google Scholar] [CrossRef]
- Lin, C.H.; Sheu, J.Y.; Wu, H.L.; Huang, Y.L. Determination of hydroquinone in cosmetic emulsion using microdialysis sampling coupled with high-performance liquid chromatography. J. Pharm. Biomed. 2005, 38, 414–419. [Google Scholar] [CrossRef]
- Gao, W.H.; Legido-Quigley, C. Fast and sensitive high performance liquid chromatography analysis of cosmetic creams for hydroquinone, phenol and six preservatives. J. Chromatogr. A 2011, 1218, 4307–4311. [Google Scholar] [CrossRef]
- Zhao, L.J.; Lv, B.Q.; Yuan, H.Y.; Zhou, Z.D.; Xiao, D. A sensitive chemiluminescence method for determination of hydroquinone and catechol. Sensors 2007, 7, 578–588. [Google Scholar] [CrossRef] [Green Version]
- Moini, M.; Cao, P.; Bard, A.J. Hydroquinone as a buffer additive for suppression of bubbles formed by electrochemical oxidation of the ce buffer at the outlet electrode in capillary electrophoresis electrospray ionisation mass spectrometry. Anal. Chem. 1999, 71, 1658–1661. [Google Scholar] [CrossRef]
- Li, M.G.; Ni, F.; Wang, Y.L.; Xu, S.D.; Zhang, D.D.; Chen, S.H.; Wang, L. Sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of resorcinol with a zn/al layered double hydroxide film modified glassy carbon electrode. Electroanalysis 2009, 21, 1521–1526. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chen, J.H.; Sun, X.; Su, Z.B.; Xing, H.T.; Hu, S.R.; Weng, W.; Guo, H.X.; Wu, W.B.; He, Y.S. One-pot hydrothermal synthesis carbon nanocages-reduced graphene oxide composites for simultaneous electrochemical detection of catechol and hydroquinone. Sens. Actuat. B Chem. 2015, 212, 165–173. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, J.; Yue, S.Z.; Zhang, L.X.; Wang, Z.H.; Guo, P.R.; Liu, Q.Y. Nickel oxide/carbon nanotube nanocomposites prepared by atomic layer deposition for electrochemical sensing of hydroquinone and catechol. J. Electroanal. Chem. 2018, 808, 245–251. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Mihailciuc, C.; Balan, A.; Nichita, C.; Stamatin, I.; Ciucu, A.A. Screen-printed prussian blue modified electrode for simultaneous detection of hydroquinone and catechol. Sens. Actuat. B Chem. 2014, 203, 824–832. [Google Scholar] [CrossRef]
- Prasad, B.B.; Madhuri, R.; Tiwari, M.P.; Sharma, P.S. Electrochemical sensor for folic acid based on a hyperbranched molecularly imprinted polymer-immobilized sol-gel-modified pencil graphite electrode. Sens. Actuat. B Chem. 2010, 146, 321–330. [Google Scholar] [CrossRef]
- Ozcan, L.; Sahin, M.; Sahin, Y. Electrochemical preparation of a molecularly imprinted polypyrrole-modified pencil graphite electrode for determination of ascorbic acid. Sensors 2008, 8, 5792–5805. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.A.; Kawde, A.N. Gold nanoparticle-modified graphite pencil electrode for the high-sensitivity detection of hydrazine. Talanta 2013, 115, 214–221. [Google Scholar] [CrossRef]
- Levent, A.; Yardim, Y.; Senturk, Z. Voltammetric behavior of nicotine at pencil graphite electrode and its enhancement determination in the presence of anionic surfactant. Electrochim. Acta 2009, 55, 190–195. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Tahernejad-Javazmi, F.; Atar, N.; Lutfi, M.; Gupta, V.K.; Ensafi, A.A. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind. Eng. Chem. Res. 2015, 54, 3634–3639. [Google Scholar] [CrossRef]
- Xu, F.C.; Ren, S.B.; Gu, Y.S. A novel conductive poly(3,4-ethylenedioxythiophene)-bsa film for the construction of a durable hrp biosensor modified with nanoau particles. Sensors 2016, 16, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.C.; Ren, S.B.; Li, J.S.; Bi, X.; Gu, Y.S. Molecular assembly of a durable hrp-aunps/pedot:Bsa/pt biosensor with detailed characterizations. Sensors 2018, 18, 1823. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.S.; Tseng, P.Y.; Bi, X.; Yang, J.H.C. Quantification of DNA by a thermal-durable biosensor modified with conductive poly(3,4-ethylenedioxythiophene). Sensors 2018, 18, 3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthika, A.; Raja, V.R.; Karuppasamy, P.; Suganthi, A.; Rajarajan, M. A novel electrochemical sensor for determination of hydroquinone in water using fewo4/sno2 nanocomposite immobilized modified glassy carbon electrode. Arab. J. Chem. 2020, 13, 4065–4081. [Google Scholar] [CrossRef]
- Li, J.S.; Bi, X.; Tamulevicius, S.; Erts, D.; Chang, C.F.; Gu, Y.S. Fabrication of a biocompatible and continuous glucose biosensor with the poly(3,4-ethylenedioxythiophene) modified electrode. J. Taiwan Inst. Chem. Eng. 2019, 104, 1–7. [Google Scholar] [CrossRef]
- Gu, Y.; Lai, M.T. The potential application of a poly(3,4-ethylenedioxythiopene) modified platinum DNA biosensor in mutation analysis. Biosens. Bioelectron. 2012, 31, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Monge-Romero, I.C.; Suarez-Herrera, M.F. Electrocatalysis of the hydroquinone/benzoquinone redox couple at platinum electrodes covered by a thin film of poly(3,4-ethylenedioxythiophene). Synth. Met. 2013, 175, 36–41. [Google Scholar] [CrossRef]
- Ling, J.L.W.; Khan, A.; Saad, B.; Ab Ghani, S. Electro polymerized 4-vinyl pyridine on 2b pencil graphite as ionophore for cadmium (ii). Talanta 2012, 88, 477–483. [Google Scholar] [CrossRef]
- Vishnu, N.; Gandhi, M.; Badhulika, S.; Kumar, A.S. Tea quality testing using 6b pencil lead as an electrochemical sensor. Anal. Methods 2018, 10, 2327–2336. [Google Scholar] [CrossRef]
- Li, C.; Imae, T. Electrochemical and optical properties of the poly(3,4-ethylenedioxythiophene) film electropolymerized in an aqueous sodium dodecyl sulfate and lithium tetrafluoroborate medium. Macromolecules 2004, 37, 2411–2416. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Chemical synthesis of pedot-au nanocomposite. Nanoscale Res. Lett. 2007, 2, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.Y.; Yang, M.; Liu, Q.Y.; Wang, X.J.; Fa, H.B.; Wang, Y.Z.; Hou, C.J. An ultrasensitive electrochemical sensor based on multiwalled carbon nanotube@reduced graphene oxide nanoribbon composite for simultaneous determination of hydroquinone, catechol and resorcinol. J. Electrochem. Soc. 2019, 166, B547–B553. [Google Scholar] [CrossRef]
- Tehrani, P.; Kanciurzewska, A.; Crispin, X.; Robinson, N.D.; Fahlman, M.; Berggren, M. The effect of ph on the electrochemical over-oxidation in pedot:Pss films. Solid State Ion. 2007, 177, 3521–3527. [Google Scholar] [CrossRef]
- Chen, T.; Friedman, K.A.; Lei, I.; Heller, A. In situ assembled mass-transport controlling micromembranes and their application in implanted amperometric glucose sensors. Anal. Chem. 2000, 72, 3757–3763. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhong, Y.M.; Zhang, Y.Y.; Weng, W.; Li, S.X. Carbon quantum dots/octahedral cu2o nanocomposites for non-enzymatic glucose and hydrogen peroxide amperometric sensor. Sens. Actuat. B Chem. 2015, 206, 735–743. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Groenendaal, B.L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Wang, J.; Fang, B.S.; Chou, K.Y.; Chen, C.C.; Gu, Y.S. A two-stage enzymatic synthesis of conductive poly(3,4-ethylenedioxythiophene). Enzym. Microb. Tech. 2014, 54, 45–50. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y. Extraction and characterizations of enzymatically synthesized conductive poly(3,4-ethylenedioxythiophene). J. Taiwan Inst. Chem. Eng. 2014, 45, 1170–1175. [Google Scholar] [CrossRef]
- Chen, T.P.; Gu, Y.S. Green chemical process for the synthesis of conductive poly(3,4-ethylenedioxythiophene) by nonthermal plasma-activated hydrogen peroxide. Plasma Process. Polym. 2020, 17, 153. [Google Scholar] [CrossRef]
- Chen, T.P.; Lin, Y.C.; Bi, X.; Gu, Y.S. Conductive poly(3,4-ethylenedioxythiophene) is effectively degradable by hydrogen peroxide with iron (ii) chloride. Mater. Chem. Phys. 2020, 242, 122509. [Google Scholar] [CrossRef]
- Donahue, M.J.; Sanchez-Sanchez, A.; Inal, S.; Qu, J.; Owens, R.M.; Mecerreyes, D.; Malliaras, G.G.; Martin, D.C. Tailoring pedot properties for applications in bioelectronics. Mat. Sci. Eng. R Res. 2020, 140, 100546. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.Y.; Yuk, H.; Lin, S.T.; Jian, N.N.; Qu, K.; Xu, J.K.; Zhao, X.H. Pure pedot:Pss hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Nie, W.Y.; Tsai, S.H.; Wang, N.X.; Huang, H.H.; Cheng, Y.J.; Wen, R.J.; Ma, L.J.; Yan, F.; Xia, Y.G. Pedot: PSS for flexible and stretchable electronics: Modifications, strategies, and applications. Adv. Sci 2019, 6, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Panigrahy, S.; Kandasubramanian, B. Polymeric thermoelectric pedot: PSS & composites: Synthesis, progress, and applications. Eur Polym J. 2020, 132, 109726. [Google Scholar]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Berchmans, S.; Venkatesan, M.; Vusa, C.S.R.; Arumugam, P. PAMAM dendrimer modified reduced graphene oxide postfunctionalized by horseradish peroxidase for biosensing H2O2. Methods Enzymol. 2018, 609, 143–170. [Google Scholar]
- Schlesinger, O.; Alfonta, L. Encapsulation of microorganisms, enzymes, and redox mediators in graphene oxide and reduced graphene oxide. Methods Enzymol. 2018, 609, 197–219. [Google Scholar]
- Shamkhalichenar, H.; Choi, J.W. Review-non-enzymatic hydrogen peroxide electrochemical sensors based on reduced graphene oxide. J. Electrochem. Soc. 2020, 167, 7531. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Torrinha, A.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Araújo, A.N. Biosensing based on pencil graphite electrodes. Talanta 2018, 190, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Prasertying, P.; Yamkesorn, M.; Chimsaard, K.; Thepsuparungsikul, N.; Chaneam, S.; Kalcher, K.; Chaisuksant, R. Modified pencil graphite electrode as a low-cost glucose sensor. J. Sci. Adv. Mater. Dev. 2020, 5, 330–336. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.-Y.; Yu, Y.-S.; Chen, T.-B.; Chang, C.-F.; Tamulevičius, S.; Erts, D.; Wu, K.C.-W.; Gu, Y. Fabrication of an Extremely Cheap Poly(3,4-ethylenedioxythiophene) Modified Pencil Lead Electrode for Effective Hydroquinone Sensing. Polymers 2021, 13, 343. https://doi.org/10.3390/polym13030343
Lu J-Y, Yu Y-S, Chen T-B, Chang C-F, Tamulevičius S, Erts D, Wu KC-W, Gu Y. Fabrication of an Extremely Cheap Poly(3,4-ethylenedioxythiophene) Modified Pencil Lead Electrode for Effective Hydroquinone Sensing. Polymers. 2021; 13(3):343. https://doi.org/10.3390/polym13030343
Chicago/Turabian StyleLu, Jian-Yu, Yu-Sheng Yu, Tung-Bo Chen, Chiung-Fen Chang, Sigitas Tamulevičius, Donats Erts, Kevin C.-W. Wu, and Yesong Gu. 2021. "Fabrication of an Extremely Cheap Poly(3,4-ethylenedioxythiophene) Modified Pencil Lead Electrode for Effective Hydroquinone Sensing" Polymers 13, no. 3: 343. https://doi.org/10.3390/polym13030343