Polymer Based Bioadhesive Biomaterials for Medical Application—A Perspective of Redefining Healthcare System Management
Abstract
:1. Introduction
1.1. Notion of Biomaterials
1.2. Overview of Bioadhesion
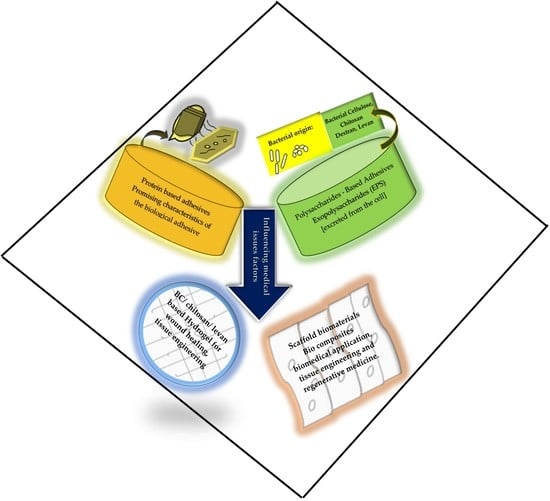
2. Bioadhesion of Biomaterials
2.1. Polysaccharides-Based Adhesives
2.2. Protein-Based Adhesives
3. Bioadhesive Biomaterials’ Biomedical Applications
4. Implementation of Bioadhesive Biomaterials in Healthcare
5. Redefining Healthcare Management in Relation to Bioadhesive Biomaterials’ Medical Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Putera, I. Redefining Health: Implication for Value-Based Healthcare Reform. Cureus 2017, 9, 1067. [Google Scholar] [CrossRef] [Green Version]
- Peled, H.B.; Pinhas, M.D. Bioadhesion and Biomimetics: From Nature to Applications; Pan Stanford: Boca Raton, FL, USA, 2015; 314p. [Google Scholar]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Definitions in Biomaterials; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Brahmbhatt, D. Bioadhesive drug delivery systems: Overview and recent advances. Int. J. Chem. Life Sci. 2017, 6, 2016–2024. [Google Scholar] [CrossRef] [Green Version]
- Palacio, M.L.B.; Bhushan, B. Bioadhesion: A review of concepts and applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 2321–2347. [Google Scholar] [CrossRef]
- Sunarintyas, S. Bioadhesion of Biomaterials. In Biomaterials and Medical Devices; Mahyudin, F., Hermawan, H., Eds.; Springer: Cham, Switzerland, 2016; Volume 58, pp. 103–125. [Google Scholar]
- Manuel, L.; Palacio, B.; Bhushan, B. Bioadhesion: A review of concepts and applications. Phil. Trans. R. Soc. A 2011, 370, 2321–2347. [Google Scholar] [CrossRef]
- Zubay, G.L. Biochemistry, 4th ed.; W.C. Brown: Dubuque, IA, USA, 1998. [Google Scholar]
- Brown, A.J. On an Acetic Ferment which form Cellulose. J. Chem. Soc. 1986, 49, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Lestari, P.; Elfrida, N.; Suryani, A.; Suryadi, Y. Study on the Production of Bacterial Cellulose from Acetobacter Xylinum Using Agro—Waste. Jordan J. Biol. Sci. 2014, 7, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Saha, N.; Vyroubal, R.; Sáha, P. Apple Juice: An alternative feed-stock to enhance the production of Bacterial Nano Cellulose. In Proceedings of the 2nd International Symposium on Bacterial Nanocellulose, Gdańsk, Poland, 9–11 September 2015. [Google Scholar]
- Zandraa, O.; Saha, N.; Shimoga, G.D.; Palem, R.R.; Saha, P. Bacterial Cellulose, An excellent biobased polymer produced from Apple, Book of Abstract Juice. In Proceedings of the 9th International Conference on Modification, Degradation and Stabilization of Polymers, Krakow, Poland, 4–8 September 2016. [Google Scholar]
- Bandopadhyay, S.; Saha, N.; Zandraa, O.; Saha, P. Bacterial cellulose from apple juice—A polysaccharide based bioadditive for sustainable food packaging, Abstract Book, 35–36. In Proceedings of the 5th EPNOE International Polysaccharide Conference, Jena, Germany, 20–24 August 2017. [Google Scholar]
- MohammadKazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Gardner, J.D.; Oporto, S.G.; Mills, R.; Samir, A.S.A.M. Adhesion and surface Issues in Cellulose and Nanaocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef] [Green Version]
- Oner, E.T.; Hernández, L.; Combie, J. Review of Levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Versluys, M.; Kirtel, O.; Oner, E.T.; Ende, W.V.D. The fructan syndrome: Evolutionary aspects and common themes among plants and microbes. Plant Cell Environ. 2018, 41, 16–38. [Google Scholar] [CrossRef] [Green Version]
- Poli, A.; Kazak, H.; Gürleyendağ, B.; Tommonaro, G.; Pieretti, G.; Oner, E.T.; Nicolaus, B. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr. Polym. 2009, 78, 651–657. [Google Scholar] [CrossRef]
- Kazak, H.; Barbosa, A.M.; Baregzay, B.; da Cunha, M.A.A.; Oner, E.T.; Dekker, R.F.H.; Khaper, N. Biological activities of bacterial levan and three fungal β-glucans, botryosphaeran and lasiodiplodan under high glucose condition in the pancreatic β-cell line INS-1E. Adapt. Biol. Med. New Dev. 2014, 7, 105–115. [Google Scholar]
- Queiroz, E.A.; Fortes, Z.B.; Da Cunha, M.A.; Sarilmiser, H.K.; Barbosa, A.M.; Oner, E.T.; Dekker, R.F.; Khaper, N. Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. Int. J. Biol. Macromol. 2017, 102, 565–570. [Google Scholar] [CrossRef]
- Sarilmiser, H.K.; Oner, E.T.; Sarılmışer, H.K. Investigation of anti-cancer activity of linear and aldehyde-activated levan from Halomonas smyrnensis AAD6T. Biochem. Eng. J. 2014, 92, 28–34. [Google Scholar] [CrossRef]
- Sezer, A.D.; Sarılmışer, H.K.; Rayaman, E.; Çevikbaş, A.; Öner, E.T.; Akbuğa, J. Development and characterization of vancomycin-loaded levan-based microparticular system for drug delivery. Pharm. Dev. Technol. 2017, 22, 627–634. [Google Scholar] [CrossRef]
- Sezer, A.D.; Kazak, H.; Oner, E.T.; Akbuğa, J. Levan-based nanocarrier system for peptide and protein drug delivery: Optimization and influence of experimental parameters on the nanoparticle characteristics. Carbohydr. Polym. 2011, 84, 358–363. [Google Scholar] [CrossRef]
- Costa, R.R.; Neto, A.I.; Calgeris, I.; Correia, C.R.; De Pinho, A.C.M.; Fonseca, J.C.; Oner, E.T.; Mano, J.F. Adhesive nanostructured multilayer films using a bacterial exopolysaccharide for biomedical applications. J. Mater. Chem. B 2013, 1, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Bostan, M.S.; Mutlu, E.C.; Kazak, H.; Keskin, S.S.; Oner, E.T.; Eroglu, M.S. Comprehensive characterization of chitosan/PEO/levan ternary blend films. Carbohydr. Polym. 2014, 102, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Oner, E.T.; Eroglu, M.S. Novel levan and pNIPA temperature sensitive hydrogels for 5-ASA controlled release. Carbohydr. Polym. 2017, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Axente, E.; Sima, F.; Sima, L.E.; Erginer, M.; Eroğlu, M.S.; Serban, N.; Ristoscu, C.; Petrescu, S.M.; Oner, E.T.; Mihailescu, I.N. Combinatorial MAPLE gradient thin film assemblies signalling to human osteoblasts. Biofabrication 2014, 6, 035010. [Google Scholar] [CrossRef] [PubMed]
- Sima, F.; Axente, E.; Sima, L.E.; Tuyel, U.; Eroǧlu, M.S.; Serban, N.; Ristoscu, C.; Petrescu, S.M.; Oner, E.T.; Mihailescu, I.N. Combinatorial matrix-assisted pulsed laser evaporation: Single-step synthesis of biopolymer compositional gradient thin film assemblies. Appl. Phys. Lett. 2012, 101, 233705. [Google Scholar] [CrossRef]
- Avsar, G.; Agirbasli, D.; Agirbasli, M.A.; Gunduz, O.; Oner, E.T. Levan based fibrous scaffolds electrospun via co-axial and single-needle techniques for tissue engineering applications. Carbohydr. Polym. 2018, 193, 316–325. [Google Scholar] [CrossRef]
- Erginer, M.; Akcay, A.; Coskunkan, B.; Morova, T.; Rende, D.; Bucak, S.; Baysal, N.; Ozisik, R.; Eroglu, M.S.; Agirbasli, M.; et al. Sulfated levan from Halomonas smyrnensis as a bioactive, heparin-mimetic glycan for cardiac tissue engineering applications. Carbohydr. Polym. 2016, 149, 289–296. [Google Scholar] [CrossRef]
- Gomes, T.D.; Caridade, S.G.; Sousa, M.P.; Azevedo, S.; Kandur, M.Y.; Öner, E.T.; Alves, N.M.; Mano, J.F. Adhesive free-standing multilayer films containing sulfated levan for biomedical applications. Acta Biomater. 2018, 69, 183–195. [Google Scholar] [CrossRef]
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of Chitin and Chitosan from Honeybees. Appl. Biochem. Microbiol. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Yao, K.; Li, J.; Yao, F.; Yin, Y. (Eds.) Chitosan-Based Hydrogels: Functions and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Vrabič Brodnjak, U. Influence of ultrasonic treatment on properties of bio-based coated paper. Prog. Org. Coat. 2017, 103, 93–100. [Google Scholar] [CrossRef]
- Vrabič Brodnjak, U. Improvement of physical and optical properties of chitosan-rice starch films pre-treated with ultrasound. Bulg. Chem. Commun. 2017, 49, 859–867. [Google Scholar]
- Mati-Baouche, N.; Elchinger, P.H.; De Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Kurek, M.; Brachais, C.H.; Ščetar, M.; Voilley, A.; Galić, K.; Couvercelle, J.P.; Debeaufort, F. Carvacrol affects interfacial, structural and transfer properties of chitosan coatings applied onto polyethylene. Carbohydr. Polym. 2013, 97, 217–225. [Google Scholar] [CrossRef]
- Bajaj, M.; Winter, J.; Gallert, C. Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochem. Eng. J. 2011, 56, 51–62. [Google Scholar] [CrossRef]
- Yanqiao, J.; Cheng, X.; Zheng, Z. Preparation and characterization of phenol–formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresour. Technol. 2010, 101, 2046–2048. [Google Scholar]
- Norström, E.; Fogelström, L.; Nordqvist, P.; Khabbaz, F.; Malmström, E. Gum dispersions as environmentally friendly wood adhesives. Ind. Crop. Prod. 2014, 52, 736. [Google Scholar] [CrossRef]
- Patel, A.K. Chitosan: Emergence as potent candidate for green adhesive market. Biochem. Eng. J. 2015, 102, 74–81. [Google Scholar] [CrossRef]
- Vrabič Brodnjak, U. Experimental investigation of novel curdlan/chitosan coatings on packaging paper. Prog. Org. Coat. 2017, 112, 86–92. [Google Scholar] [CrossRef]
- Richter, K.; Grunwald, I.; von Byern, J. Bioadhesives; da Silva, L.F.M., Oechsner, A., Adams, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–45. [Google Scholar]
- Von Byern, J.; Grunwald, I. Biological Adhesive Systems: From Nature to Technical and Medical Application; Springer: New York, NY, USA, 2010. [Google Scholar]
- Von Byern, J.; Müller, C.; Voigtländer, K.; Dorrer, V.; Marchetti-Deschmann, M.; Flammang, P.; Mayer, G. Examples of Bioadhesives for Defence and Predation; Gorb, S., Gorb, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 141–191. [Google Scholar]
- Ferguson, J.; Nürnberger, S.; Redl, H. Fibrin: The Very First Biomimetic Glue—Still a Great Tool; von Byern, J., Grunwald, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 225–236. [Google Scholar]
- Nürnberger, S.; Wolbank, S.; Peterbauer, A.; Morton, T.J.; Feichtinger, G.A.; Gugerell, A.; Meinl, A.; Labuda, K.; Bittner, M.; Pasteiner, W.; et al. Properties and Potential Alternative Applications of Fibrin Glue; von Byern, J., Grunwald, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 237–259. [Google Scholar]
- Bell, E.; Gosline, J. Mechanical design of mussel byssus: Material yield enhances attachment strength. J. Exp. Biol. 1996, 199, 1005–1017. [Google Scholar]
- Harrington, M.J.; Waite, J.H. Holdfast heroics: Comparing the molecular and mechanical properties of Mytilus californianus byssal threads. J. Exp. Biol. 2007, 210, 4307–4318. [Google Scholar] [CrossRef] [Green Version]
- Graham, L.D. Biological Adhesives from Nature; Bowlin, G.L., Wnek, G., Eds.; Taylor & Francis: Abingdon, UK, 2005; pp. 1–18. [Google Scholar]
- Smith, A.M. Biological Adhesives; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Krogsgaard, M.; Andersen, A.; Birkedal, H. Gels and threads: Mussel-inspired one-pot route to advanced responsive materials. Chem. Commun. 2014, 50, 13278–13281. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef]
- Shen, H.; Qian, Z.; Zhao, N.; Xu, J. Preparation and Application of Biomimetic Materials Inspired by Mussel Adhesive Proteins; Yang, G., Xiao, L., Lamboni, L., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 103–118. [Google Scholar]
- Smith, A.M. The Structure and Function of Adhesive Gels from Invertebrates. Integr. Comp. Biol. 2002, 42, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M. Gastropod Secretory Glands and Adhesive Gels; von Byern, J., Grunwald, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 41–51. [Google Scholar]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I. Wet Adhesion and Adhesive Locomotion of Snails on Anti-Adhesive Non-Wetting Surfaces. PLoS ONE 2012, 7, e36983. [Google Scholar] [CrossRef]
- Bonnemain, B. Helix and Drugs: Snails for Western Health Care from Antiquity to the Present. Evid.-Based Complement. Altern. Med. 2005, 2, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Brieva, A.; Philips, N.; Tejedor, R.; Guerrero, A.; Pivel, J.; Alonso-Lebrero, J.; Gonzalez, S. Molecular Basis for the Regenerative Properties of a Secretion of the Mollusk Cryptomphalus aspersa. Ski. Pharmacol. Physiol. 2008, 21, 15–22. [Google Scholar] [CrossRef]
- La Cruz, M.C.I.-D.; Sanz-Rodríguez, F.; Zamarrón, A.; Reyes, E.; Carrasco, E.; González, S.; Juarranz, A. A secretion of the mollusc Cryptomphalus aspersa promotes proliferation, migration and survival of keratinocytes and dermal fibroblasts in vitro. Int. J. Cosmet. Sci. 2012, 34, 183–189. [Google Scholar] [CrossRef]
- Fabi, S.G.; Cohen, J.L.; Peterson, J.D.; Kiripolsky, M.G.; Goldman, M.P. The effects of filtrate of the secretion of the Cryptomphalus aspersa on photoaged skin. J. Drugs Dermatol. JDD 2013, 12, 453–457. [Google Scholar]
- Meyer-Rochow, V.B.; Yamahama, Y.A. Comparison between the larval eyes of the dimly luminescent Keroplatus nipponicus and the brightly luminescent Arachnocampa luminosa (Diptera; Keroplatidae). Luminescence 2017, 32, 1072–1076. [Google Scholar] [CrossRef]
- Tsoutsos, D.; Kakagia, D.; Tamparopoulos, K. The efficiacy of Helix aspersa Müller extract in the healing of partial thickness burns: A novel treatment for open burn management protocols. J. Dermatol. Treat. 2009, 20, 219–222. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Tyler, M.J. Adhesive Dermal Secretions of the Amphibia, with Particular Reference to the Australian Limnodynastid Genus Notaden; von Byern, J., Grunwald, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 181–186. [Google Scholar]
- Von Byern, J.; Dicke, U.; Heiss, E.; Grunwald, I.; Gorb, S.; Staedler, Y.; Cyran, N. Morphological characterization of the glue-producing system in the salamander Plethodon shermani (Caudata, Plethodontidae). Zoology 2015, 118, 334–347. [Google Scholar] [CrossRef]
- Szomor, Z.L.; Murrell, G.A.C.; Appleyard, R.C.; Tyler, M.J. Meniscal repair with a new biological glue: An ex vivo study. Tech. Knee Surg. 2009, 7, 261–265. [Google Scholar] [CrossRef]
- Von Byern, J.; Grunwald, I.; Kosok, M.; Saporito, R.A.; Dicke, U.; Wetjen, O.; Thiel, K.; Borcherding, K.; Kowalik, T.; Marchetti-Deschmann, M.; et al. Chemical characterization of the adhesive secretions of the salamander Plethodon shermani (Caudata, Plethodontidae). Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Graham, L.D.; Glattauer, V.; Huson, M.G.; Maxwell, J.M.; Knott, R.B.; White, J.W.; Vaughan, P.R.; Peng, Y.; Tyler, M.J.; Werkmeister, J.A.; et al. Characterization of a protein-based adhesive elastomer secreted by the Australian frog Notaden bennetti. Biomacromolecules 2005, 6, 3300–3312. [Google Scholar] [CrossRef]
- Graham, L.D.; Glattauer, V.; Li, D.; Tyler, M.J.; Ramshaw, J.A. The adhesive skin exudate of Notaden bennetti frogs (Anura: Limnodynastinae) has similarities to the prey capture glue of Euperipatoides sp. velvet worms (Onychophora: Peripatopsidae). Comp. Biochem. Physiol. Ser. B Biochem. Mol. Biol. 2013, 165, 250–259. [Google Scholar] [CrossRef]
- Graham, L.D.; Danon, S.J.; Johnson, G.; Braybrook, C.; Hart, N.K.; Varley, R.J.; Evans, M.; McFarland, G.A.; Tyler, M.J.; Werkmeister, J.A.; et al. Biocompatibility and modification of the protein-based adhesive secreted by the Australian frogNotaden bennetti. J. Biomed. Mater. Res. Part A 2009, 93, 429–441. [Google Scholar] [CrossRef]
- Millar, N.L.; Bradley, T.A.; Walsh, N.A.; Appleyard, R.C.; Tyler, M.J.; Murrell, G.A. Frog glue enhances rotator cuff repair in a laboratory cadaveric model. J. Shoulder Elb. Surg. 2009, 18, 639–645. [Google Scholar] [CrossRef]
- Tyler, M.J.; Ramshaw, J.A. An Adhesive Derived from Amphibian Skin Secretions. Australia Patent No. WO2002/022756, 18 September 2000. [Google Scholar]
- Von Byern, J.; Mebs, D.; Heiss, E.; Dicke, U.; Wetjen, O.; Bakkegard, K.; Grunwald, I.; Wolbank, S.; Mühleder, S.; Gugerell, A.; et al. Salamanders on the bench—A biocompatibility study of salamander skin secretions in cell cultures.et al. Salamanders on the bench—A biocompatibility study of salamander skin secretions in cell cultures. Toxicon 2017, 135, 24–32. [Google Scholar] [CrossRef]
- Undheim, E.A.B.; Fry, B.G.; King, G.F. Centipede Venom: Recent Discoveries and Current State of Knowledge. Toxins 2015, 7, 679–704. [Google Scholar] [CrossRef] [Green Version]
- Hopkin, S.P.; Anger, H.S. On the structure and function of the glue-secreting glands of Henia vesuviana (Newport, 1845) (Chilopoda: Geophilomorpha). Ber. Nat.-Med. Ver. Innsbr. Suppl. 1992, 10, 71–79. [Google Scholar]
- Hopkin, S.P. Defensive secretion of proteinaceous glues by Henia (Chaetechelyne) vesuviana (Chilopoda Geophilomorpha). In Proceedings of the 7th International Congress of Myriapodology, Brill, The Netherlands, 1 March 1990; pp. 175–181. [Google Scholar]
- Jones, T.H.; Conner, W.E.; Meinwald, J.; Eisner, H.E.; Eisner, T. Benzoyl cyanide and mandelonitrile in the cynogenetic secretion of a centipede. J. Chem. Ecol. 1976, 2, 421–429. [Google Scholar] [CrossRef]
- Maschwitz, U.; Lauschke, U.; Würmli, M. Hydrogen cyanide-producing glands in a scolopender, Asanada n.sp. (Chilopoda, Scolopendridae). J. Chem. Ecol. 1979, 5, 901–907. [Google Scholar] [CrossRef]
- Schildknecht, H.; Maschwitz, U.; Krauss, D. Blausäure im Wehrsekret des Erdläufers Pachymerium ferrugineum. Die Nat. 1968, 55, 230. [Google Scholar] [CrossRef]
- Vujisić, L.; Vučković, I.M.; Makarov, S.E.; Ilić, B.; Antić, D.Ž.; Jadranin, M.B.; Todorović, N.M.; Mrkic, I.; Vajs, V.E.; Lučić, L.R.; et al. Chemistry of the sternal gland secretion of the Mediterranean centipede Himantarium gabrielis (Linnaeus, 1767) (Chilopoda: Geophilomorpha: Himantariidae). Naturwissenschaften 2013, 100, 861–870. [Google Scholar] [CrossRef]
- Rathi, S.; Saka, R.; Domb, A.J.; Khan, W. Protein-based bioadhesives and bioglues. Polym. Adv. Technol. 2018, 1–18. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic Potential of Chitosan and Its Derivatives in Regenerative Medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef]
- Qasim, S.S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratakis, E. Novel Biomaterials for Tissue Engineering 2018. Int. J. Mol. Sci. 2018, 19, 3960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Zheng, C.; Ning, Z.X.; Yang, B. Modification of Low Molecular Weight Polysaccharides from Tremella Fuciformis and Their Antioxidant Activity In Vitro. Int. J. Mol. Sci. 2007, 8, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Larsson, T.F.; Martín Martínez, J.M.; Vallés, J.L. Biomaterials for Healthcare. In A Decade of EU-Funded Research; Office for Official Publications of the European Communities: Luxembourg, 2007. [Google Scholar]
- Prodan, A.M.; Andronescu, E.; Truşcă, R.; Beuran, M.; Iconaru, S.L.; Barna, E.Ş.; Chifiriuc, M.C.; Marutescu, L. Anti-biofilm Activity of Dextran Coated Iron Oxide Nanoparticles. Univ. Politeh. Buchar. Sci. Bull. Ser. B Chem. Mater. Sci. 2014, 76, 81–90. [Google Scholar]
- Iconaru, S.L.; Turculet, C.S.; Coustumer, P.L.; Bleotu, C.; Chifiriuc, M.; Lazar, V.; Surugiu, A.; Badea, M.; Iordache, F.; Soare, M.; et al. Biological Studies on Dextrin Coated Iron Oxide Nanoparticles. Rom. Rep. Phys. 2016, 68, 1536–1544. [Google Scholar]
- Gale, A.J. Current Understanding of Hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Mehdizadeh, M.; Yang, J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Value-Based Healthcare: A Global Assessment; The Economist Intelligence Unit: London, UK, 2016.
- Petrova, M.; Dale, J.; Fulford, B.K.W.M. Values-based practice in primary care: Easing the tensions between individual values, ethical principles and best evidence. Br. J. Gen. Pract. 2006, 56, 703–709. [Google Scholar]
- Badash, I.; Kleinman, N.P.; Barr, S.; Jang, J.; Rahman, S.; Wu, B.W. Redefining Health: The Evolution of Health Ideas from Antiquity to the Era of Value-Based Care. Cureus 2017, 9, e1018. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, R. Evaluating state wide disease management programs. Regenstrief Institute for Healthcare. In Proceedings of the AHRQ Medicaid Care Management Learning Network, Rockville, NY, USA, 2 October 2006. [Google Scholar]
- Designing and Implementing Medicaid Disease and Care Management Programs; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2014. Available online: https://www.ahrq.gov/patient-safety/settings/long-term-care/resource/hcbs/medicaidmgmt/index.html (accessed on 25 November 2020).




| Polymer-Based Bioadhesive Biomaterials | Medical Applications |
|---|---|
| Bacterial Cellulose (BC) | Drug delivery, wound dressing, implantable devices (Scaffold) and BC-based biomaterials’ medical applications in bone, skin and cardiovascular tissue engineering. |
| Chitosan | Tissue engineering and a promising substitute for regenerative medicine as a bioactive polymer. |
| Levan | Surgical bandages and sealants and in tissue engineering mainly contributing to promoting and controlling specific cellular responses related to their adhesion, and wound healing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, N.; Saha, N.; Sáha, T.; Toksoy Öner, E.; Brodnjak, U.V.; Redl, H.; von Byern, J.; Sáha, P. Polymer Based Bioadhesive Biomaterials for Medical Application—A Perspective of Redefining Healthcare System Management. Polymers 2020, 12, 3015. https://doi.org/10.3390/polym12123015
Saha N, Saha N, Sáha T, Toksoy Öner E, Brodnjak UV, Redl H, von Byern J, Sáha P. Polymer Based Bioadhesive Biomaterials for Medical Application—A Perspective of Redefining Healthcare System Management. Polymers. 2020; 12(12):3015. https://doi.org/10.3390/polym12123015
Chicago/Turabian StyleSaha, Nibedita, Nabanita Saha, Tomas Sáha, Ebru Toksoy Öner, Urška Vrabič Brodnjak, Heinz Redl, Janek von Byern, and Petr Sáha. 2020. "Polymer Based Bioadhesive Biomaterials for Medical Application—A Perspective of Redefining Healthcare System Management" Polymers 12, no. 12: 3015. https://doi.org/10.3390/polym12123015