Preparations of Tough and Conductive PAMPS/PAA Double Network Hydrogels Containing Cellulose Nanofibers and Polypyrroles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of TEMPO-Oxidized Cellulose Nanofibers (TOCNs)
2.3. Preparation of Single Network Hydrogels Included with TOCN (i.e., T–SN Hydrogel)
2.4. Preparation of Double Network Hydrogels Included with TOCN (i.e., T–DN Hydrogels)
2.5. Preparation of Double Network Hydrogels Hybridized with TOCN and Py (i.e., from T–Fe–DN to T–Py–DN Hydrogels)
2.6. Determination of the Carboxylate Content (CC) and Degree of Oxidation (DO) of TOCN
2.7. Estimations of Water Content and Degree of Swelling for Hydrogels
2.8. Establishment of the Calibration Curve of Fe(III)EDTA Solution and Estimations of Fe3+ Concentration Absorbed in Hydrogels
2.9. Characterization
3. Results and Discussion
3.1. Preparations of TOCN and Hybridizations with Double Network (i.e., T–DN) Hydrogels
3.2. Incorporations of Various Fe3+ Concentrations into T–DN Hydrogels
3.3. Characteristic of Functional Groups within Hydrogels
3.4. Microstructures of DN Hydrogels
3.5. Thermal Stability of T–Py–DN Hydrogels
3.6. Mechanical Properties of T–Py–DN Hydrogels
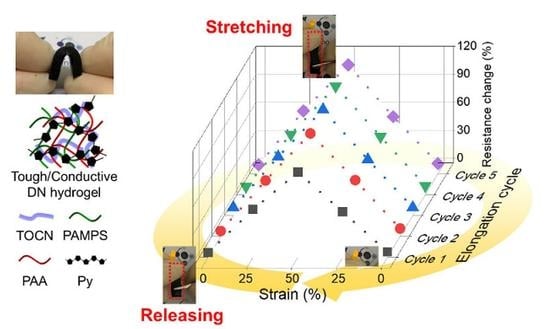
3.7. Electrical Conductivity (Σ) and Resistivity (ρ) of T–Py–DN Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Kumbar, S.G.; Rudzinski, W. Stimulus-responsive “smart” hydrogels as novel drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent hydrogels that are robust and highly stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.J.; Doroudiani, S. Superabsorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2010, 32, 277–289. [Google Scholar] [CrossRef]
- Kabiri, K.; Zohuriaan-Mehr, M.J. Superabsorbent hydrogel composites. Polym. Adv. Technol. 2003, 14, 438–444. [Google Scholar] [CrossRef]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nat. Cell Biol. 2000, 404, 588–590. [Google Scholar] [CrossRef]
- Burdick, J.A.; Khademhosseini, A.; Langer, R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir 2004, 20, 5153–5156. [Google Scholar] [CrossRef]
- Wu, C.-H.; Tu, C.-W.; Aimi, J.; Zhang, J.-W.; Chen, T.; Wang, C.-C.; Huang, C.-F. Mechanochromic double network hydrogels as a compression stress sensor. Polym. Chem. 2020, 11, 6423–6428. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Butt, H.; Volpatti, L.R.; Pavlichenko, I.; Humar, M.; Kwok, S.J.; Koo, H.; Kim, K.S.; Naydenova, I.; Khademhosseini, A.; et al. Photonic hydrogel sensors. Biotechnol. Adv. 2016, 34, 250–271. [Google Scholar] [CrossRef] [Green Version]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Desai, M.S.; Lee, S.-W. Light-controlled graphene-elastin composite hydrogel actuators. Nano Lett. 2013, 13, 2826–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, X.; Lu, W.; Zhang, J.; Chen, T. Recent progress in biomimetic anisotropic hydrogel actuators. Adv. Sci. 2019, 6, 1801584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Liu, R.; Liang, S.; Tang, X.-Z.; Yan, D.; Li, X.; Yu, Z.-Z. Tough and highly stretchable graphene oxide/polyacrylamide nanocomposite hydrogels. J. Mater. Chem. 2012, 22, 14160–14167. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.-J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef]
- Hu, J.; Hiwatashi, K.; Kurokawa, T.; Liang, S.M.; Wu, Z.L.; Gong, J.P. Microgel-reinforced hydrogel films with high mechanical strength and their visible mesoscale fracture structure. Macromolecules 2011, 44, 7775–7781. [Google Scholar] [CrossRef]
- Hu, J.; Kurokawa, T.; Hiwatashi, K.; Nakajima, T.; Wu, Z.L.; Liang, S.M.; Gong, J.P. Structure optimization and mechanical model for microgel-reinforced hydrogels with high strength and toughness. Macromolecules 2012, 45, 5218–5228. [Google Scholar] [CrossRef]
- Haque, A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Fan, C.; Liao, L.; Zhang, C.; Liu, L. A tough double network hydrogel for cartilage tissue engineering. J. Mater. Chem. B 2013, 1, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT: PSS hydrogels. Nat. Commun. 2019, 10, 1–10. [Google Scholar]
- Spencer, A.R.; Primbetova, A.; Koppes, A.N.; Koppes, R.A.; Fenniri, H.; Annabi, N. Electroconductive gelatin methacryloyl-PEDOT:PSS composite hydrogels: Design, synthesis, and properties. ACS Biomater. Sci. Eng. 2018, 4, 1558–1567. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Lin, D.; Ni, Y.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Biocompatible, self-wrinkled, antifreezing and stretchable hydrogel-based wearable sensor with PEDOT:sulfonated lignin as conductive materials. Chem. Eng. J. 2019, 370, 1039–1047. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Zhong, W.; Yang, W. Hydrothermal direct synthesis of polyaniline, graphene/polyaniline and N-doped graphene/polyaniline hydrogels for high performance flexible supercapacitors. J. Mater. Chem. A 2018, 6, 9245–9256. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Chen, Y.; Li, C.-L.; Tang, J.; Li, Q. Synthesis of three-dimensional nitrogen-doped graphene/polyaniline hydrogels for high performance supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 10674–10683. [Google Scholar] [CrossRef]
- Zhou, K.; He, Y.; Xu, Q.; Zhang, Q.; Zhou, A.; Lu, Z.; Yang, L.-K.; Jiang, Y.; Ge, D.; Liu, X.Y.; et al. A Hydrogel of ultrathin pure polyaniline nanofibers: Oxidant-templating preparation and supercapacitor application. ACS Nano 2018, 12, 5888–5894. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Wei, D.; Lin, X.; Li, L.; Shang, S.; Yuen, M.C.-W.; Yan, G.; Yu, X. Controlled growth of polypyrrole hydrogels. Soft Matter 2013, 9, 2832–2836. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive hydrogels as smart materials for flexible electronic devices. Chem. A Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Yu, G.; Peng, L.; Yu, G. Nanostructured conducting polymer hydrogels for energy storage applications. Nanoscale 2015, 7, 12796–12806. [Google Scholar] [CrossRef]
- Lin, F.; Zheng, R.; Chen, J.; Su, W.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Microfibrillated cellulose enhancement to mechanical and conductive properties of biocompatible hydrogels. Carbohydr. Polym. 2019, 205, 244–254. [Google Scholar] [CrossRef]
- Kuttel, M.M.; Naidoo, K.J. Free energy surfaces for the α (1→4)-glycosidic linkage: Implications for polysaccharide solution structure and dynamics. J. Phys. Chem. B 2005, 109, 7468–7474. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Liu, H.; Du, C.; Yu, H.; Zhou, Z. Thermo-responsive and compression properties of TEMPO-oxidized cellulose nanofiber-modified PNIPAm hydrogels. Carbohydr. Polym. 2016, 147, 201–207. [Google Scholar] [CrossRef]
- Sultana, T.; Van Hai, H.; Abueva, C.; Kang, H.J.; Lee, S.-Y.; Lee, B.-T. TEMPO oxidized nano-cellulose containing thermo-responsive injectable hydrogel for post-surgical peritoneal tissue adhesion prevention. Mater. Sci. Eng. C 2019, 102, 12–21. [Google Scholar] [CrossRef]
- Isobe, N.; Chen, X.; Kim, U.-J.; Kimura, S.; Wada, M.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose hydrogel as a high-capacity and reusable heavy metal ion adsorbent. J. Hazard. Mater. 2013, 260, 195–201. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S.-H. Novel chitosan-cellulose nanofiber self-healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019, 11, 25. [Google Scholar] [CrossRef]
- Huang, C.-F.; Tu, C.-W.; Lee, R.-H.; Yang, C.-H.; Hung, W.-C.; Lin, K.-Y.A. Study of various diameter and functionality of TEMPO-oxidized cellulose nanofibers on paraquat adsorptions. Polym. Degrad. Stab. 2019, 161, 206–212. [Google Scholar] [CrossRef]
- Endo, R.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose nanofibril/poly(vinyl alcohol) composite drawn fibers. Polymer 2013, 54, 935–941. [Google Scholar] [CrossRef]
- Tu, C.-W.; Tsai, F.-C.; Chang, C.-J.; Yang, C.-H.; Kuo, S.-W.; Zhang, J.-W.; Chen, T.; Huang, C.-F. Surface-Initiated Initiators for Continuous Activator Regeneration (SI ICAR) ATRP of MMA from 2,2,6,6-tetramethylpiperidine-1-oxy (TEMPO) Oxidized Cellulose Nanofibers for the Preparations of PMMA Nanocomposites. Polymers 2019, 11, 1631. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.-D.; Huang, C.-F.; Hsu, S.-H. Composites of waterborne polyurethane and cellulose nanofibers for 3D printing and bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Huang, C.-F. Data in support of dual-functionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Data Brief. 2015, 3, 195–200. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.; Chen, G.; Wu, J. Rapid shape memory TEMPO-oxidized cellulose nanofibers/polyacrylamide/gelatin hydrogels with enhanced mechanical strength. Carbohydr. Polym. 2017, 171, 77–84. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Zhan, Y.; Liu, B.; Xiong, C.; Yang, Q.; Hu, G.-H. Fe3+ Cross-linked polyaniline/cellulose nanofibril hydrogels for high-performance flexible solid-state supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 17653–17660. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, J.-K.; Tsai, T.-Y.; Hsieh, Y.-A.; Lin, K.-Y.A. Dual-functionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Polymer 2015, 72, 395–405. [Google Scholar] [CrossRef]
- Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Vimala, K.; Raju, K.M. Synthesis and characterizations of macroporous poly(acrylamide-2-acrylamido-2-methyl-1-propanesulfonic acid) hydrogels for in vitro drug release of ranitidine hydrochloride. Int. J. Polym. Mater. 2011, 60, 490–503. [Google Scholar] [CrossRef]
- Zhang, C.; Easteal, A.J. Thermoanalytical, spectroscopic, and morphological study of poly(ethylene glycol)/poly(2-acrylamido-2-methylpropanesulfonic acid-co-N-isopropylacrylamide) semi-interpenetrating network gels. J. Appl. Polym. Sci. 2007, 104, 1723–1731. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. FTIR studies of conformational energies of poly(acrylic acid) in cast films. J. Polym. Sci., Part. B Polym. Phys. 1997, 35, 507–515. [Google Scholar] [CrossRef]
- Ibrahim, I.; Yunus, S.; Hashim, M. Relative performance of isopropylamine, pyrrole and pyridine as corrosion inhibitors for carbon steels in saline water at mildly elevated temperatures. Int. J. Sci. Eng. Res. 2013, 4, 1–12. [Google Scholar]
- Zhang, X.; Wu, X.; Lu, C.; Zhou, Z. Dialysis-free and in situ doping synthesis of polypyrrole@cellulose nanowhiskers nanohybrid for preparation of conductive nanocomposites with enhanced properties. ACS Sustain. Chem. Eng. 2015, 3, 675–682. [Google Scholar] [CrossRef]









| Sample | TOCN Content (wt.%) | [FeCl3]0 (mM) | Water Content (wt.%) | Degree of Swelling |
|---|---|---|---|---|
| T0–DN | 0 | – | 94.2 | 16.4 |
| T0–Py5–DN | 0 | 5 | 93.5 | 14.6 |
| T1–Py5–DN | 1 | 5 | 92.7 | 12.8 |
| T2–Py5–DN | 2 | 5 | 94.4 | 16.2 |
| T3–Py5–DN | 3 | 5 | 93.2 | 13.8 |
| T2–Py5(16h)–DN * | 2 | 5 | 94.9 | 18.7 |
| T2–Py50–DN | 2 | 50 | 93.6 | 14.8 |
| T2–Py100–DN | 2 | 100 | 92.0 | 11.6 |
| T2–Py200–DN | 2 | 200 | 91.7 | 11.3 |
| Sample | Conc. of Fe3+ in DN Hydrogels (mM) |
|---|---|
| T2–DN | 0 |
| T2–Fe5–DN | 3.68 |
| T2–Fe5(16h)–DN | 8.20 |
| T2–Fe50–DN | 6.97 |
| T2–Fe100–DN | 9.38 |
| T2–Fe200–DN | 20.67 |
| Sample | Td,5% (°C) | Char Yield (wt.%) | Conductivity (×10−3 S/cm) | Resistivity * (Ω × cm) |
|---|---|---|---|---|
| T0–DN | 232 | 2.1 | 0.17 | 5930 |
| T0–Py5–DN | 228 | 1.6 | 0.26 | 3893 |
| T1–Py5–DN | 232 | 2.1 | 1.72 | 583 |
| T2–Py5–DN | 237 | 1.8 | 3.34 | 299 |
| T3–Py5–DN | 233 | 1.3 | 2.37 | 431 |
| T2–Py5(16h)–DN | 237 | 1.8 | 2.21 | 454 |
| T2–Py50–DN | 241 | 2.5 | 1.74 | 594 |
| T2–Py100–DN | 237 | 3.6 | 1.22 | 823 |
| T2–Py200–DN | 238 | 3.9 | 1.37 | 736 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, C.-W.; Tsai, F.-C.; Chen, J.-K.; Wang, H.-P.; Lee, R.-H.; Zhang, J.; Chen, T.; Wang, C.-C.; Huang, C.-F. Preparations of Tough and Conductive PAMPS/PAA Double Network Hydrogels Containing Cellulose Nanofibers and Polypyrroles. Polymers 2020, 12, 2835. https://doi.org/10.3390/polym12122835
Tu C-W, Tsai F-C, Chen J-K, Wang H-P, Lee R-H, Zhang J, Chen T, Wang C-C, Huang C-F. Preparations of Tough and Conductive PAMPS/PAA Double Network Hydrogels Containing Cellulose Nanofibers and Polypyrroles. Polymers. 2020; 12(12):2835. https://doi.org/10.3390/polym12122835
Chicago/Turabian StyleTu, Cheng-Wei, Fang-Chang Tsai, Jem-Kun Chen, Huei-Ping Wang, Rong-Ho Lee, Jiawei Zhang, Tao Chen, Chung-Chi Wang, and Chih-Feng Huang. 2020. "Preparations of Tough and Conductive PAMPS/PAA Double Network Hydrogels Containing Cellulose Nanofibers and Polypyrroles" Polymers 12, no. 12: 2835. https://doi.org/10.3390/polym12122835