Hydrophilicity Improvement of Polymer Surfaces Induced by Simultaneous Nuclear Transmutation and Oxidation Effects Using High-Energy and Low-Fluence Helium Ion Beam Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. He Ion Beam Irradiation
2.3. Characterization
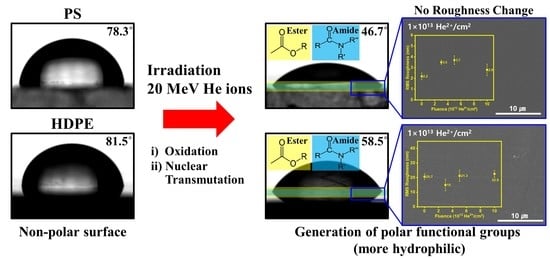
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsai, M.Y.; Lin, J.C. Surface characterization and platelet adhesion studies of self-assembled monolayer with phosphonate ester and phosphonic acid functionalities. J. Biomed. Mater. Res. 2001, 55, 554–565. [Google Scholar] [CrossRef]
- Bain, C.D.; Whitesides, G.M. Molecular-level control over surface order in self-assembled monolayer films of thiols on gold. Science 1988, 240, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Kohno, Y.; Nakamura, N.; Ohno, H. Introduction of hydrophilic groups onto the ortho-position of benzoate anions induced phase separation of the corresponding ionic liquids with water. Chem. Commun. 2013, 49, 10248–10250. [Google Scholar] [CrossRef]
- He, Q.; Liu, Z.; Xiao, P.; Liang, R.; He, N.; Lu, Z. Preparation of hydrophilic poly (dimethylsiloxane) stamps by plasma-induced grafting. Langmuir 2003, 19, 6982–6986. [Google Scholar] [CrossRef]
- Rembaum, A.; Yen, S.; Molday, R. Synthesis and reactions of hydrophilic functional microspheres for immunological studies. J. Macromol. Sci. Chem. 1979, 13, 603–632. [Google Scholar] [CrossRef]
- Alcântara, M.; Lincopan, N.; Santos, P.; Ramirez, P.; Brant, A.; Riella, H.; Lugão, A. Simultaneous hydrogel crosslinking and silver nanoparticle formation by using ionizing radiation to obtain antimicrobial hydrogels. Radiat. Phys. Chem. 2019, 165, 108369. [Google Scholar] [CrossRef]
- Al-Ajlony, A.; Tripathi, J.; Hassanein, A. Low energy helium ion irradiation induced nanostructure formation on tungsten surface. J. Nucl. Mater. 2017, 488, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.T.; Chen, W.L.; Fan, P.W.; Yao, I.C. Effect of thermal annealing on the surface properties of electrospun polymer fibers. Macromol. Rapid Commun. 2014, 35, 360–366. [Google Scholar] [CrossRef]
- Cáceres, C.A.; Mazzola, N.; França, M.; Canevarolo, S.V. Controlling in-line the energy level applied during the corona treatment. Polym. Test. 2012, 31, 505–511. [Google Scholar] [CrossRef]
- Vlaeva, I.; Yovcheva, T.; Viraneva, A.; Kitova, S.; Exner, G.; Guzhova, A.; Galikhanov, M. Contact angle analysis of corona treated polypropylene films. J. Phys. Conf. Ser. 2012, 398, 012054. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Best, J.P.; Shorubalko, I.; Michler, J.; Spolenak, R.; Wheeler, J.M. Influence of helium ion irradiation on the structure and strength of diamond. Carbon 2020, 158, 337–345. [Google Scholar] [CrossRef]
- Nanda, G.; Goswami, S.; Watanabe, K.; Taniguchi, T.; Alkemade, P.F. Defect Control and n-Doping of Encapsulated Graphene by Helium-Ion-Beam Irradiation. Nano Lett. 2015, 15, 4006–4012. [Google Scholar] [CrossRef] [PubMed]
- Abrams, K.; Hinks, J.; Pawley, C.; Greaves, G.; Van Den Berg, J.; Eyidi, D.; Ward, M.; Donnelly, S. Helium irradiation effects in polycrystalline Si, silica, and single crystal Si. J. Appl. Phys. 2012, 111, 083527. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; Denhartog, J.; Eriksson, L.; Mayer, J. Ion implantation of silicon: I. atom location and lattice disorder by means of 1.0-mev helium ion scattering. Can. J. Phys. 1967, 45, 4053–4071. [Google Scholar] [CrossRef]
- Palei, M.; Motapothula, M.; Ray, A.; Abdelhady, A.L.; Lanzano, L.; Prato, M.; Panda, J.K.; Scarpellini, A.; Pellegrini, V.; Primetzhofer, D. Photoluminescence enhancement and high accuracy patterning of lead halide perovskite single crystals by MeV ion beam irradiation. J. Mater. Chem. C 2020, 8, 9923–9930. [Google Scholar] [CrossRef]
- Li, S.; Fan, Y.; Chen, H.; Nie, J.; Liang, Y.; Tao, X.; Zhang, J.; Chen, X.; Fu, E.; Wang, Z.L. Manipulating the triboelectric surface charge density of polymers by low-energy helium ion irradiation/implantation. Energy Environ. Sci. 2020, 13, 896–907. [Google Scholar] [CrossRef]
- Gelamo, R.V.; Durrant, S.F.; Trasferetti, B.C.; Davanzo, C.U.; Rouxinol, F.P.; Bica de Moraes, M.A. Helium Ion Irradiation of Polymer Films Deposited from TMS-Ar Plasmas. Plasma Process. Polym. 2007, 4, 489–496. [Google Scholar] [CrossRef]
- Park, J.-W.; Lee, E.H.; Lee, J.-S.; Lee, B.-H.; Kim, M.-K.; Lee, C.-Y.; Kim, H.-J.; Choi, B.-H. Dual ion beam irradiation of polymeric materials for the modification of optical properties with improved adhesion. Nucl. Instrum. Methods Phys. Res. Sect. B 2012, 281, 51–55. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Biersack, J.P. The stopping and range of ions in matter. In Treatise on Heavy-Ion Science; Springer: Boston, MA, USA, 1985; pp. 93–129. [Google Scholar]
- Starchyk, M.; Marchenko, L.; Pinkovska, M.; Shmatko, G.; Varnina, V. Voids’ layer structures in silicon irradiated with high doses of high-energy helium ions. Semicond. Phys. Quantum Electron. Optoelectron. 2015, 18, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Yoshida, N.; Iwakiri, H.; Xu, Z. Thermal desorption and surface modification of He+ implanted into tungsten. J. Nucl. Mater. 2004, 329, 692–696. [Google Scholar] [CrossRef]
- Dukes, C.; Baragiola, R.; McFadden, L. Surface modification of olivine by H+ and He+ bombardment. J. Geophys. Res. Planets 1999, 104, 1865–1872. [Google Scholar] [CrossRef]
- Tanyeli, I.; Marot, L.; van de Sanden, M.C.; De Temmerman, G. Nanostructuring of iron surfaces by low-energy helium ions. ACS Appl. Mater. Interfaces 2014, 6, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Carrez, P.; Demyk, K.; Cordier, P.; Gengembre, L.; Grimblot, J.; D’hendecourt, L.; Jones, A.P.; Leroux, H. Low-energy helium ion irradiation-induced amorphization and chemical changes in olivine: Insights for silicate dust evolution in the interstellar medium. Meteorit. Planet. Sci. 2002, 37, 1599–1614. [Google Scholar] [CrossRef]
- Clough, R.; Gillen, K. Polymer degradation under ionizing radiation: The role of ozone. J. Polym. Sci. Part A 1989, 27, 2313–2324. [Google Scholar] [CrossRef]
- Ahad, I.U.; Butruk, B.; Ayele, M.; Budner, B.; Bartnik, A.; Fiedorowicz, H.; Ciach, T.; Brabazon, D. Extreme ultraviolet (EUV) surface modification of polytetrafluoroethylene (PTFE) for control of biocompatibility. Nucl. Instrum. Methods Phys. Res. Sect. B 2015, 364, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Steen, M.L.; Butoi, C.I.; Fisher, E.R. Identification of Gas-Phase Reactive Species and Chemical Mechanisms Occurring at Plasma—Polymer Surface Interfaces. Langmuir 2001, 17, 8156–8166. [Google Scholar] [CrossRef]
- Tompkins, B.D.; Dennison, J.M.; Fisher, E.R. Etching and Post-Treatment Surface Stability of Track-Etched Polycarbonate Membranes by Plasma Processing Using Various Related Oxidizing Plasma Systems. Plasma Process. Polym. 2014, 11, 850–863. [Google Scholar] [CrossRef]
- Karasawa, H.; Ibe, E.; Uchida, S.; Etoh, Y.; Yasuda, T. Radiation induced decomposition of nitrogen. Int. J. Radiat. Appl. Instrum. Part C 1991, 37, 193–197. [Google Scholar] [CrossRef]
- Bartnik, A.; Lisowski, W.; Sobczak, J.; Wachulak, P.; Budner, B.; Korczyc, B.; Fiedorowicz, H. Simultaneous treatment of polymer surface by EUV radiation and ionized nitrogen. Appl. Phys. A 2012, 109, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Hayden, H.C.; Utterback, N.G. Ionization of helium, neon, and nitrogen by helium atoms. Phys. Rev. 1964, 135, A1575. [Google Scholar] [CrossRef]
- Moran, T.; Conrads, R. Charge neutralization of He+ ion beams. J. Chem. Phys. 1973, 58, 3793–3799. [Google Scholar] [CrossRef]
- Kusakabe, T.; Miyamoto, Y.; Kimura, M.; Tawara, H. Charge-transfer processes in collisions of He2+ ions with H2, N2, O2, CO, and CO2 molecules below 4 keV/u. Phys. Rev. A 2006, 73, 022706. [Google Scholar] [CrossRef]








| # | Nuclear Reaction | Threshold Energy (MeV) | Decay Mode, Half-life |
|---|---|---|---|
| (1) | 12C (α, n)15O → 15N | 11.338 | β+, 122.24 s |
| (2) | 12C (α, p)15N | 0 | |
| (3) | 13C (α, n)16O | 0 | |
| (4) | 13C (α, p)16N → 16O | 9.708 | β−, 7.13 s |
| (5) | 13C (α, 2n)15O → 15N | 17.588 | β+, 122.24 s |
| (6) | 13C (α, np)15N | 12.962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Yoo, S.H.; Kong, Y.B.; Cho, S.O.; Lee, E.J. Hydrophilicity Improvement of Polymer Surfaces Induced by Simultaneous Nuclear Transmutation and Oxidation Effects Using High-Energy and Low-Fluence Helium Ion Beam Irradiation. Polymers 2020, 12, 2770. https://doi.org/10.3390/polym12122770
Kim JW, Yoo SH, Kong YB, Cho SO, Lee EJ. Hydrophilicity Improvement of Polymer Surfaces Induced by Simultaneous Nuclear Transmutation and Oxidation Effects Using High-Energy and Low-Fluence Helium Ion Beam Irradiation. Polymers. 2020; 12(12):2770. https://doi.org/10.3390/polym12122770
Chicago/Turabian StyleKim, Jung Woo, Seung Hwa Yoo, Young Bae Kong, Sung Oh Cho, and Eun Je Lee. 2020. "Hydrophilicity Improvement of Polymer Surfaces Induced by Simultaneous Nuclear Transmutation and Oxidation Effects Using High-Energy and Low-Fluence Helium Ion Beam Irradiation" Polymers 12, no. 12: 2770. https://doi.org/10.3390/polym12122770