Preparation and Characterization of Electrospun Double-layered Nanocomposites Membranes as a Carrier for Centella asiatica (L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethanol Extraction of Crude Centella Asiatica (CA) Plant
2.3. Minimum Inhibitory Concentration (MIC) of the Crude CA extract
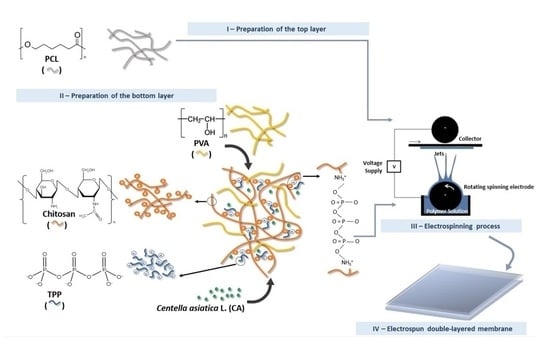
2.4. Fabrication of the Double-Layered Nanocomposites Membranes
2.5. Characterization of the Produced Double-Layered Nanocomposites Membranes
2.5.1. Scanning Electron Microscopy (SEM) Imaging and Analysis
2.5.2. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy Study
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Assessment of the Mechanical Characteristics of the Produced Double-Layered Nanocomposites Membranes
2.5.5. Measurement of the Total Porosity
2.5.6. Evaluation of Wettability Properties
2.5.7. Analysis of the In Vitro Swelling Behavior
2.5.8. Study of the In Vitro Biodegradation Profile
2.5.9. Water Vapor Transmission Rate (WVTR) Analysis
2.6. Analysis of the In Vitro CA Release from Double-Layered Nanocomposites Membranes
2.7. Assessment of the Antibacterial Properties of the Produced Double-Layered Nanocomposites Membranes
2.8. Analysis of the In Vitro Cell Viability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Minimal Inhibitory Concentration (MIC) of the Crude CA Extract
3.2. Characterization of the Produced Double-Layered Nanocomposites Membranes
3.2.1. Scanning Electron Microscopy (SEM) Imaging and Analysis
3.2.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy Study
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. Assessment of the Mechanical Characteristics of the Produced Electrospun Double-Layered Nanocomposites Membranes
3.2.5. Measurement of the Total Porosity
3.2.6. Evaluation of Wettability Properties
3.2.7. Analysis of the In Vitro Swelling Behavior
3.2.8. Study of the In Vitro Biodegradation Profile
3.2.9. Water Vapor Transmission Rate (WVTR) Analysis
3.3. Analysis of the In Vitro CA Release from Electrospun Double-Layered Nanocomposites Membranes
3.4. Assessment of the Antibacterial Properties of the Produced Electrospun Double-Layered Nanocomposites Membranes
3.5. Analysis of the In Vitro Cell Viability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sousa Coelho, D.; Veleirinho, B.; Alberti, T.; Maestri, A.; Yunes, R.; Fernando Dias, P.; Maraschin, M. Electrospinning Technology: Designing Nanofibers toward Wound Healing Application. In Nanomaterials—Toxicity, Human Health and Environment; IntechOpen: London, UK, 2018; pp. 1–19. [Google Scholar]
- Williamson, D.; Harding, K. Wound healing. Medicine 2004, 32, 4–7. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarheed, O.; Ahmed, A.; Shouqair, D.; Boateng, J. Antimicrobial Dressings for Improving Wound Healing. In Wound Healing—New Insights into Ancient Challenges; InTech: London, UK, 2016; pp. 373–398. [Google Scholar]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [Green Version]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Rev. Med. Chem. 2017, 18, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, D.M.; Leite, I.S.; de Lacerda Bukzem, A.; de Oliveira Santos, R.P.; Frollini, E.; Inada, N.M.; Campana-Filho, S.P. Nanostructured electrospun nonwovens of poly(ε-caprolactone)/quaternized chitosan for potential biomedical applications. Carbohydr. Polym. 2018, 186, 110–121. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun polycaprolactone/Aloe Vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, D.; Zhang, Z.; Pan, J.; Cui, Z.; Yu, D.-G.; Annie Bligh, S.-W. Testing of fast dissolution of ibuprofen from its electrospun hydrophilic polymer nanocomposites. Polym. Test. 2020, 106872. [Google Scholar] [CrossRef]
- Wang, K.; Wang, P.; Wang, M.; Yu, D.G.; Wan, F.; Bligh, S.W.A. Comparative study of electrospun crystal-based and composite-based drug nano depots. Mater. Sci. Eng. C 2020, 113, 110988. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Zhang, K.; Gong, Q.; Yu, D.G.; Wang, J.; Tan, X.; Quan, H. Ethylcellulose-based drug nano depots fabricated using a modified triaxial electrospinning. Int. J. Biol. Macromol. 2020, 152, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Liu, P.; Chen, X.; Yu, D.G. Electrospun multiple-chamber nanostructure and its potential self-healing applications. Polymers 2020, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.G.; Liu, Z.; Guo, J.Z. Electrospun Janus zein–PVP nanofibers provide a two-stage controlled release of poorly water-soluble drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- Hou, J.; Yang, J.; Zheng, X.; Wang, M.; Liu, Y.; Yu, D.G. A nanofiber-based drug depot with high drug loading for sustained release. Int. J. Pharm. 2020, 583. [Google Scholar] [CrossRef]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.G.; Liu, Y.; Shao, J. Core-shell eudragit S100 nanofibers preparedvia triaxial electrospinning to providea colon-targeted extended drug release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Chang, S.; Wang, M.; Zhang, F.; Liu, Y.; Liu, X.; Yu, D.G.; Shen, H. Sheath-separate-core nanocomposites fabricated using a trifluid electrospinning. Mater. Des. 2020, 192, 108782. [Google Scholar] [CrossRef]
- Hassanin, A.; El-Moneim, A.A.; Ghaniem, M.; Nageh, H. Nanocomposite multilayer fibrous membrane for sustained drug release. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2014; Volume 894, pp. 364–368. [Google Scholar]
- Rezk, A.I.; Lee, J.Y.; Son, B.C.; Park, C.H.; Kim, C.S. Bi-layered nanofibers membrane loaded with titanium oxide and tetracycline as controlled drug delivery system for wound dressing applications. Polymers 2019, 11, 1602. [Google Scholar] [CrossRef] [Green Version]
- López-Calderón, H.D.; Avilés-Arnaut, H.; Galán-Wong, L.J.; Almaguer-Cantú, V.; Laguna-Camacho, J.R.; Calderón-Ramón, C.; Escalante-Martínez, J.E.; Arévalo-Niño, K. Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing. Polymers 2020, 12, 2311. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.K.; Buttar, H.S. Perspectives on nanofiber dressings for the localized delivery of botanical remedies in wound healing. AIMS Mater. Sci. 2017, 4, 370–382. [Google Scholar] [CrossRef]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Suryamathi, M.; Ruba, C.; Viswanathamurthi, P.; Balasubramanian, V.; Perumal, P. Tridax Procumbens Extract Loaded Electrospun PCL Nanofibers: A Novel Wound Dressing Material. Macromol. Res. 2019, 27, 55–60. [Google Scholar] [CrossRef]
- Foong, C.Y.; Sultana, N. Fabrication of layer-by-layer electrospun composite membranes based on polylactic acid (PLA) and poly (caprolactone) (PCL)/Chitosan. ARPN J. Eng. Appl. Sci. 2015, 10, 9408–9413. [Google Scholar]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Preparation of chitosan-thiamine pyrophosphate/polyvinyl alcohol blend electrospun nanofibers. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; Volume 506, pp. 118–121. [Google Scholar]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: An experimental animal study. BMC Complement. Altern. Med. 2012, 12, 103. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Krishnan, L.; Bharadvaja, N. Qualitative and Quantitative Phytochemical Analysis of Centella asiatica. Nat. Prod. Chem. Res. 2018, 6, 1000323. [Google Scholar] [CrossRef]
- Sikareepaisan, P.; Suksamrarn, A.; Supaphol, P. Electrospun gelatin fiber mats containing a herbal—Centella asiatica—Extract and release characteristic of asiaticoside. Nanotechnology 2008, 19, 015102. [Google Scholar] [CrossRef]
- Yousefi, I.; Pakravan, M.; Rahimi, H.; Bahador, A.; Farshadzadeh, Z.; Haririan, I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaspour, M.; Makhmalzadeh, B.S.; Rezaee, B.; Shoja, S.; Ahangari, Z. Evaluation of the antimicrobial effect of chitosan/polyvinyl alcohol electrospun nanofibers containing mafenide acetate. Jundishapur J. Microbiol. 2015, 8, e24239. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.D.; Farrugia, B.L.; Dargaville, T.R.; Dhara, S. Physico-chemical/biological properties of tripolyphosphate cross-linked chitosan based nanofibers. Mater. Sci. Eng. C 2013, 33, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2017, 43, 3527–3537. [Google Scholar] [CrossRef]
- Yeh, C.C.; Li, Y.T.; Chiang, P.H.; Huang, C.H.; Wang, Y.; Chang, H.I. Characterizing microporous PCL matrices for application of tissue engineering. J. Med. Biol. Eng. 2009, 29, 92–97. [Google Scholar]
- Kaur, I.; Suthar, N.; Kaur, J.; Bansal, Y.; Bansal, G. Accelerated Stability Studies on Dried Extracts of Centella asiatica Through Chemical, HPLC, HPTLC, and Biological Activity Analyses. J. Evid.-Based Complement. Altern. Med. 2016, 21, NP127–NP137. [Google Scholar] [CrossRef]
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 2015, 11, 905–915. [Google Scholar] [CrossRef]
- Pourhojat, F.; Sohrabi, M.; Shariati, S.; Mahdavi, H.; Asadpour, L. Evaluation of poly ε-caprolactone electrospun nanofibers loaded with Hypericum perforatum extract as a wound dressing. Res. Chem. Intermed. 2017, 43, 297–320. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Bolhuis-Versteeg, L.A.M.; Grijpma, D.W.; Feijen, J.; Wessling, M.; Stamatialis, D. A facile method to fabricate poly(L-lactide) nano-fibrous morphologies by phase inversion. Acta Biomater. 2010, 6, 2477–2483. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Manotham, S.; Pengpat, K.; Eitssayeam, S.; Rujijanagul, G.; Sweatman, D.R.; Tunkasiri, T. Fabrication of Polycaprolactone/Centella asiatica Extract Biopolymer Nanofiber by Electrospinning. Appl. Mech. Mater. 2015, 804, 151–154. [Google Scholar] [CrossRef]
- Amina, M.; Al-Youssef, H.M.; Amna, T.; Hassan, S.; El-Shafae, A.M.; Kim, H.Y.; Khil, M.-S. Poly(urethane)/G. Mollis Composite Nanofibers for Biomedical Applications. J. Nanoeng. Nanomanuf. 2012, 2, 85–90. [Google Scholar] [CrossRef]
- Rebia, R.A.; Sadon, N.S.B.; Tanaka, T. Natural antibacterial reagents (Centella, propolis, and hinokitiol) loaded into poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] composite nanofibers for biomedical applications. Nanomaterials 2019, 9, 1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Emulsion electrospinning of polycaprolactone: Influence of surfactant type towards the scaffold properties. J. Biomater. Sci. Polym. Ed. 2015, 26, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Mirzadeh, H.; Shokrgozar, M.A.; Farokhi, M. Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 2015, 5, 10479–10487. [Google Scholar] [CrossRef]
- Vega-Cázarez, C.A.; López-Cervantes, J.; Sánchez-Machado, D.I.; Madera-Santana, T.J.; Soto-Cota, A.; Ramírez-Wong, B. Preparation and Properties of Chitosan–PVA Fibers Produced by Wet Spinning. J. Polym. Environ. 2018, 26, 946–958. [Google Scholar] [CrossRef]
- Ghaseminezhad, K.; Zare, M.; Lashkarara, S.; Yousefzadeh, M.; Aghazadeh Mohandesi, J. Fabrication of althea officinalis loaded electrospun nanofibrous scaffold for potential application of skin tissue engineering. J. Appl. Polym. Sci. 2020, 137, 48587. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Preparation and characterization of Calendula officinalis-loaded PCL/gum arabic nanocomposite scaffolds for wound healing applications. Iran. Polym. J. 2019, 28, 51–63. [Google Scholar] [CrossRef]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(ε-caprolactone)/polystyrene blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Trinca, R.B.; Westin, C.B.; da Silva, J.A.F.; Moraes, Â.M. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar] [CrossRef]
- Wang, J.; Planz, V.; Vukosavljevic, B.; Windbergs, M. Multifunctional electrospun nanofibers for wound application—Novel insights into the control of drug release and antimicrobial activity. Eur. J. Pharm. Biopharm. 2018, 129, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef]
- Salvatore, L.; Carofiglio, V.E.; Stufano, P.; Bonfrate, V.; Calò, E.; Scarlino, S.; Nitti, P.; Centrone, D.; Cascione, M.; Leporatti, S.; et al. Potential of electrospun poly(3-hydroxybutyrate)/collagen blends for tissue engineering applications. J. Healthc. Eng. 2018, 2018, 6573947. [Google Scholar] [CrossRef]
- Eğri, Ö.; Erdemir, N. Production of Hypericum perforatum oil-loaded membranes for wound dressing material and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Orue, I.; Santos-Vizcaino, E.; Etxabide, A.; Uranga, J.; Bayat, A.; Guerrero, P.; Igartua, M.; de la Caba, K.; Hernandez, R.M. Development of bioinspired gelatin and gelatin/chitosan bilayer hydrofilms for wound healing. Pharmaceutics 2019, 11, 314. [Google Scholar] [CrossRef] [Green Version]
- Goh, Y.-F.; Shakir, I.; Hussain, R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013, 48, 3027–3054. [Google Scholar] [CrossRef]
- Avci, H.; Ghorbanpoor, H.; Nurbas, M. Preparation of origanum minutiflorum oil-loaded core–shell structured chitosan nanofibers with tunable properties. Polym. Bull. 2018, 75, 4129–4144. [Google Scholar] [CrossRef]
- Hou, Q.; Li, M.; Lu, Y.H.; Liu, D.H.; Li, C.C. Burn wound healing properties of asiaticoside and madecassoside. Exp. Ther. Med. 2016, 12, 1269–1274. [Google Scholar] [CrossRef] [Green Version]







| Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Thickness (mm) | |
|---|---|---|---|---|
| PCL/PVA_CS-TPP | 3.96 ± 0.99 | 38.16 ± 5.32 | 10.39 ± 1.89 | 0.12 ± 0.01 |
| PCL/PVA_CS-TPP_CA | 3.03 ± 0.67 | 36.36 ± 7.29 | 8.31 ± 0.61 | 0.10 ± 0.02 |
| Native skin | 2.50–30.00 a | 0.40–20.00 a | 10.00–115.00 a | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouro, C.; Fangueiro, R.; Gouveia, I.C. Preparation and Characterization of Electrospun Double-layered Nanocomposites Membranes as a Carrier for Centella asiatica (L.). Polymers 2020, 12, 2653. https://doi.org/10.3390/polym12112653
Mouro C, Fangueiro R, Gouveia IC. Preparation and Characterization of Electrospun Double-layered Nanocomposites Membranes as a Carrier for Centella asiatica (L.). Polymers. 2020; 12(11):2653. https://doi.org/10.3390/polym12112653
Chicago/Turabian StyleMouro, Cláudia, Raul Fangueiro, and Isabel C. Gouveia. 2020. "Preparation and Characterization of Electrospun Double-layered Nanocomposites Membranes as a Carrier for Centella asiatica (L.)" Polymers 12, no. 11: 2653. https://doi.org/10.3390/polym12112653