Development of Hybrid and Templated Silica-P123 Membranes for Brackish Water Desalination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sol–Gel Synthesis and Characterization
2.2. Membrane Fabrication and Testing
3. Results and Discussion
3.1. Membrane Characteristics
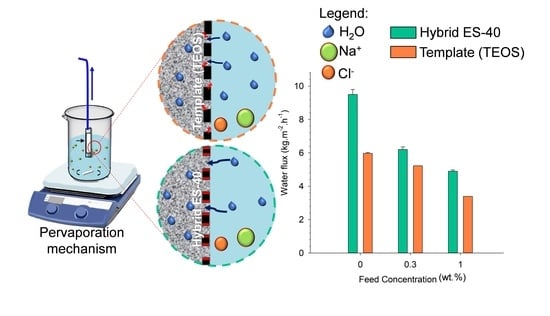
3.2. Membrane Pervaporation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rich, V.I.; Maier, R.M. Chapter 6—Aquatic Environments. In Environmental Microbiology, 3rd ed.; Pepper, I.L., Gerba, C.P., Gentry, T.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 111–138. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Arifin, S.N.H.M.; Hizam, S.M.; Rampun, E.L.A.; Bilad, M.R.; Elma, M.; Khan, A.L.; Wibisono, Y.; Jaafar, J. Chlorella vulgaris broth harvesting via standalone forward osmosis using seawater draw solution. Bioresour. Technol. Rep. 2020, 9, 100394. [Google Scholar] [CrossRef]
- Choi, W.; Jeon, S.; Kwon, S.J.; Park, H.; Park, Y.-I.; Nam, S.-E.; Lee, P.S.; Lee, J.S.; Choi, J.; Hong, S. Thin film composite reverse osmosis membranes prepared via layered interfacial polymerization. J. Membr. Sci. 2017, 527, 121–128. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Handayani, N. The Performance of Membranes Interlayer-Free Silica-Pectin Templated for Seawater Desalination via Pervaporation Operated at High Temperature of Feed Solution. Mater. Sci. Forum 2020, 981, 349–355. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Luis, P. Chapter Four—Pervaporation. In Progress in Filtration and Separation; Tarleton, S., Ed.; Academic Press: Oxford, UK, 2015; pp. 101–154. [Google Scholar] [CrossRef]
- Darmawan, A.; Motuzas, J.; Smart, S.; Julbe, A.; Diniz da Costa, J.C. Binary iron cobalt oxide silica membrane for gas separation. J. Membr. Sci. 2015, 474, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.K.; Diniz da Costa, J.C.; Smart, S. Development of rapid thermal processing of tubular cobalt oxide silica membranes for gas separations. J. Membr. Sci. 2014, 456, 192–201. [Google Scholar] [CrossRef]
- Miller, C.R.; Wang, D.K.; Smart, S.; Da Costa, J.C.D. Reversible redox effect on gas permeation of cobalt doped ethoxy polysiloxane (ES40) membranes. Sci. Rep. 2013, 3, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; Diniz da Costa, J.C. Binary gas mixture and hydrothermal stability investigation of cobalt silica membranes. J. Membr. Sci. 2015, 493, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, D.K.; Kappen, P.; Martens, D.L.; Smart, S.; Diniz da Costa, J.C. Hydrothermal stability investigation of micro- and mesoporous silica containing long-range ordered cobalt oxide clusters by XAS. Phys. Chem. Chem. Phys. 2015, 17, 19500–19506. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; Strounina, E.; Diniz da Costa, J.C. Physicochemical characterisation and hydrothermal stability investigation of cobalt-incorporated silica xerogels. Rsc Adv. 2014, 4, 18862–18870. [Google Scholar] [CrossRef]
- Rahman, S.K.; Maimunawaro; Rahma, A.; Syauqiah, I.; Elma, M. Functionalization of hybrid organosilica based membranes for water desalination – Preparation using Ethyl Silicate 40 and P123. Mater. Today Proc. 2020, 31, 60–64. [Google Scholar] [CrossRef]
- Maimunawaro; Karina Rahman, S.; Lulu Atika Rampun, E.; Rahma, A.; Elma, M. Deconvolution of carbon silica templated thin film using ES40 and P123 via rapid thermal processing method. Mater. Today Proc. 2020, 31, 75–78. [Google Scholar] [CrossRef]
- Diniz Da Costa, J.C. Synthesis and Characterisation of Molecular Sieve Silica (MSS) Membranes. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2000. [Google Scholar]
- Ellis, F.P.K. Fabrication of Random Hole Optical Fiber Preforms by Silica Sol-Gel Processing. Master’s Thesis, Faculty of the Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2004. [Google Scholar]
- Elma, M.; Riskawati, N.; Marhamah. Silica Membranes for Wetland Saline Water Desalination: Performance and Long Term Stability. Iop Conf. Ser. Earth Environ. Sci. 2018, 175, 012006. [Google Scholar] [CrossRef]
- Elma, M.; Ayu, R.; Rampun, E.L.A.; Annahdliyah, S.; Suparsih, D.R.; Sari, N.L.; Pratomo, D.A. Fabrication of interlayer-free silica-based membranes—Effect of low calcination temperature using an organo-catalyst. Membr. Technol. 2019, 2019, 6–10. [Google Scholar] [CrossRef]
- Wijaya, S.; Duke, M.C.; Diniz da Costa, J.C. Carbonised template silica membranes for desalination. Desalination 2009, 236, 291–298. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Kanezashi, M.; Asaeda, M. Hydrogen permeation characteristics and stability of Ni-doped silica membranes in steam at high temperature. J. Membr. Sci. 2006, 271, 86–93. [Google Scholar] [CrossRef]
- Darmawan, A.; Karlina, L.; Astuti, Y.; Sriatun; Wang, D.K.; Motuzas, J.; da Costa, J.C.D. Interlayer free—Nickel doped silica membranes for desalination. Iop Conf. Ser. Mater. Sci. Eng. 2017, 172, 012001. [Google Scholar] [CrossRef]
- Darmawan, A.; Karlina, L.; Astuti, Y.; Sriatun; Motuzas, J.; Wang, D.K.; da Costa, J.C.D. Structural evolution of nickel oxide silica sol-gel for the preparation of interlayer-free membranes. J. Non-Cryst. Solids 2016, 447, 9–15. [Google Scholar] [CrossRef]
- Smart, S.; Vente, J.F.; da Costa, J.C.D. High temperature H2/CO2 separation using cobalt oxide silica membranes. Int. J. Hydrog. Energy 2012, 37, 12700–12707. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; Diniz da Costa, J.C. Interlayer-free microporous cobalt oxide silica membranes via silica seeding sol–gel technique. J. Membr. Sci. 2015, 492, 1–8. [Google Scholar] [CrossRef]
- Elma, M.; Saputro, G.S. Performance of Cobalt-Silica Membranes through Pervaporation Process with Different Feed Solution Concentrations. Mater. Sci. Forum 2020, 981, 342–348. [Google Scholar] [CrossRef]
- Cornelius, C.J.; Marand, E. Hybrid inorganic–organic materials based on a 6FDA–6FpDA–DABA polyimide and silica: Physical characterization studies. Polymer 2002, 43, 2385–2400. [Google Scholar] [CrossRef]
- Wang, D.K.; Elma, M.; Motuzas, J.; Hou, W.-C.; Schmeda-Lopez, D.R.; Zhang, T.; Zhang, X. Physicochemical and photocatalytic properties of carbonaceous char and titania composite hollow fibers for wastewater treatment. Carbon 2016, 109, 182–191. [Google Scholar] [CrossRef]
- Rampun, E.L.A.; Elma, M.; Syauqiah, I.; Putra, M.D.; Rahma, A.; Pratiwi, A.E. Interlayer-free Silica Pectin Membrane for Wetland Saline Water via Pervaporation. Jurnal Kimia Sains dan Aplikasi 2019, 22, 99. [Google Scholar] [CrossRef]
- Pratiwi, A.E.; Elma, M.; Rahma, A.; Rampun, E.L.A.; Saputro, G.S. Deconvolution of pectin carbonised template silica thin-film: Synthesis and characterisation. Membr. Technol. 2019, 2019, 5–8. [Google Scholar] [CrossRef]
- Yang, H.; Elma, M.; Wang, D.K.; Motuzas, J.; da Costa, J.C.D. Interlayer-Free Hybrid Carbon-Silica Membranes for Processing Brackish to Brine Salt Solutions by Pervaporation. J. Membr. Sci. 2017, 523, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Elma, M.; Wang, D.K.; Yacou, C.; da Costa, J.C.D. Interlayer-Free P123 Carbonised Template Silica Membranes for Desalination with Reduced Salt Concentration Polarisation. J. Membr. Sci. 2015, 475, 376–383. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; da Costa, J.C.D. Interlayer-Free Hybrid Organo-Silica Membranes Based Teos and Tevs for Water Desalination. Conf. Oleo Petrochem. Eng. 2015, 49–57. [Google Scholar]
- Ibrahim, S.M.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Organosilica bis(triethoxysilyl)ethane (BTESE) membranes for gas permeation (GS) and reverse osmosis (RO): The effect of preparation conditions on structure, and the correlation between gas and liquid permeation properties. J. Membr. Sci. 2017, 526, 242–251. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.K.; Jack, K.S.; Smart, S.; Diniz da Costa, J.C. Improved hydrothermal stability of silica materials prepared from ethyl silicate 40. Rsc Adv. 2015, 5, 6092–6099. [Google Scholar] [CrossRef]
- Moreth, K.; Frey, H.; Hubo, M.; Zeng-Brouwers, J.; Nastase, M.-V.; Hsieh, L.T.-H.; Haceni, R.; Pfeilschifter, J.; Iozzo, R.V.; Schaefer, L. Biglycan-triggered TLR-2-and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 2014, 35, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Tripathi, B.M.; Kumar Ghosh, S. Chapter 4—Low Temperature Coating Deriving from Metal-Organic Precursors: An Economical and Environmentally Benign Approach. In Intelligent Coatings for Corrosion Control; Tiwari, A., Rawlins, J., Hihara, L.H., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 93–134. [Google Scholar] [CrossRef]
- Elma, M.; Setyawan, H.; Rahma, A.; Pratiwi, A.E.; Rampun, E.L.A. Fabrication of Interlayer-free P123 Caronised Template Silica Membranes for Water Desalination: Conventional Versus Rapid Thermal Processing (CTP vs RTP) Techniques. Iop Conf. Ser. Mater. Sci. Eng. 2019, 543, 012076. [Google Scholar] [CrossRef] [Green Version]
- Wang, S. High Performance es40-Derived Silica Membranes for Desalination. PhD Thesis, University of Queensland, Brisbane, Australia, 2016. [Google Scholar]
- Liu, L.; Ding, J.; Sarrigani, G.V.; Fitzgerald, P.; Aljunid Merican, Z.M.; Lim, J.-W.; Tseng, H.-H.; Xie, F.; Zhang, B.; Wang, D.K. Enhanced catalyst dispersion and structural control of Co3O4-silica nanocomposites by rapid thermal processing. Appl. Catal. B Environ. 2020, 262, 118246. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.K.; Motuzas, J.; Smart, S.; da Costa, J.C.D. Rapid thermal treatment of interlayer-free ethyl silicate 40 derived membranes for desalination. J. Membr. Sci. 2016, 516, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; Diniz da Costa, J.C. Influence of sol–gel conditioning on the cobalt phase and the hydrothermal stability of cobalt oxide silica membranes. J. Membr. Sci. 2015, 475, 425–432. [Google Scholar] [CrossRef]
- Wang, D.K.; Motuzas, J.; da Costa, J.C.D.; Smart, S. Rapid thermal processing of tubular cobalt oxide silica membranes. Int. J. Hydrog. Energy 2013, 38, 7394–7399. [Google Scholar] [CrossRef]
- Ayu Lestari, R.; Elma, M.; Rampun, E.L.A.; Sumardi, A.; Paramitha, A.; Eka Lestari, A.; Rabiah, S.; Assyaifi, Z.L.; Satriaji, G. Functionalization of Si-C Using TEOS (Tetra Ethyl Ortho Silica) as Precursor and Organic Catalyst. E3s Web Conf. 2020, 148, 07008. [Google Scholar] [CrossRef]
- Bonekamp, B.C. Chapter 6: Preparation of asymmetric ceramic membrane supports by dip-coating. In Membrane Science and Technology; Burggraaf, A.J., Cot, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 4, pp. 141–225. [Google Scholar]
- Marcos-Hernández, M.; Villagrán, D. Applications. In Composite Nanoadsorbents; Kyzas, G.Z., Mitropoulos, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–293. [Google Scholar] [CrossRef]
- Yoon, S.B.; Choi, B.-S.; Lee, K.-W.; Moon, J.-K.; Choi, Y.S.; Kim, J.-Y.; Cho, H.; Kim, J.H.; Kim, M.-S.; Yu, J.-S. New mesoporous silica/carbon composites by in situ transformation of silica template in carbon/silica nanocomposite. J. Exp. Nanosci. 2014, 9, 221–229. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.J.P.; Chemistry, A. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Zhu, J.; Huang, W.; Fang, J.; Sun, X.; Wang, X.; Liao, K. Preparation of Nano-Porous Carbon-Silica Composites and Its Adsorption Capacity to Volatile Organic Compounds. Processes 2020, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Lestari, R.A.; Elma, M.; Rahma, A.; Suparsih, D.; Anadhliyah, S.; Sari, N.L.; Pratomo, D.A.; Sumardi, A.; Lestari, A.E.; Assyaifi, Z.L.; et al. Organo Silica Membranes for Wetland Saline Water Desalination: Effect of membranes calcination temperatures. E3s Web Conf. 2020, 148, 07006. [Google Scholar] [CrossRef]
- Mrowiec-Bialon, J.; Jarzebski, A.B.; Pajak, L.; Olejniczak, Z.; Gibas, M. Preparation and surface properties of low-density gels synthesized using prepolymerized silica precursors. Langmuir 2004, 20, 10389–10393. [Google Scholar] [CrossRef]
- Mrowiec-Bialon, J.; Turek, W.; Jarzębski, A. Preparation of highly active heteropolyacid-silica composite catalysts using the SOL–GEL method. React. Kinet. Catal. Lett. 2002, 76, 213–219. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Wang, X. Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vib. Spectrosc. 2008, 46, 1–7. [Google Scholar] [CrossRef]
- More, P.M.; Umbarkar, S.B.; Dongare, M.K. Template-free sol–gel synthesis of high surface area mesoporous silica based catalysts for esterification of di-carboxylic acids. Comptes Rendus Chim. 2016, 19, 1247–1253. [Google Scholar] [CrossRef]
- Yang, H.; Wang, D.K.; Motuzas, J.; da Costa, J.C.D. Hybrid vinyl silane and P123 template sol−gel derived carbon silica membrane for desalination. J. Sol-Gel Sci. Technol. 2017, 85, 280–289. [Google Scholar] [CrossRef]
- Ladewig, B.P.; Tan, Y.H.; Lin, C.X.C.; Ladewig, K.; Diniz da Costa, J.C.; Smart, S. Preparation, Characterization and Performance of Templated Silica Membranes in Non-Osmotic Desalination. Materials 2011, 4, 845. [Google Scholar] [CrossRef]
- Lin, C.X.C.; Ding, L.P.; Smart, S.; Diniz da Costa, J.C. Cobalt oxide silica membranes for desalination. J. Colloid Interface Sci. 2012, 368, 70–76. [Google Scholar] [CrossRef]
- Duke, M.C.; Mee, S.; da Costa, J.C.D. Performance of porous inorganic membranes in non-osmotic desalination. Water Res. 2007, 41, 3998–4004. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K.; Rangarajan, R. Organic−Inorganic Hybrid Membrane: Thermally Stable Cation-Exchange Membrane Prepared by the Sol−Gel Method. Macromolecules 2004, 37, 10023–10030. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]






| Membrane Type | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) | Reference |
|---|---|---|---|---|
| TEOS-P123 templated | 572 | 0.315 | 2.21 | This work |
| ES40-P123 hybrid | 671 | 0.45 | 3.67 | This work |
| Carbonized silica membranes C16 | 793 | 0.37 | - | [19] |
| Hybrid membrane | 554 | 0.38 | 2.71 | [55] |
| Carbonized C12 silica template | 661 | 0.37 | - | [19] |
| Carbonised P123-silica template | 965 | 0.50 | 2.32 | |
| Hybrid TEVS-P123 | 922 | 0.97 | 2.10 | [56] |
| Membrane Type | Calcination Method | Feed Temperatures (°C) | NaCl Concentration (%) | Water Flux (kg·m−2·h−1) | Salt Rejection (%) | References |
|---|---|---|---|---|---|---|
| TEOS-P123 templated | RTP (air) | 25 | 0.3–1 | 5.2–3.4 | >99.2 | This work |
| ES40-P123 hybrid | RTP (air) | 25 | 0.3–1 | 6.2–4.9 | >99.8 | This work |
| Carbonized silica membranes | CTP (air) | 20 | 0.3–3.5 | 2.1–1.9 | 99.5 | [58] |
| Carbonized silica membranes | CTP (vacuum) | 20 | 0.3–3.5 | 3.2–1.4 | 89 | [19] |
| Silica-pectin membranes | RTP (air) | 25 | 3.5 | 5.73 | >99 | |
| Silica-P123 membranes | RTP (air) | 25 | 3.5 | 1.49 | 99.8 | |
| Silica membrane | RTP (air) | 26 | 0.3–15 | 2.9–1.2 | >95 | [17] |
| Hybrid membrane | CTP (vacuum) | 60–25 | 1–15 | 5.7–2.3 | >99.7 | [55] |
| Carbonised P123-silica template | CTP (vacuum) | 22 | 0.3 | 1.7 | >99.5 | |
| Carbonised C16 silica template | CTP (vacuum) | 60 | 0.3–3.5 | 3.1–2.1 | 91–97% | [19] |
| Hybrid TEVS-P123 | CTP (vacuum) | 60 | 0.3 | 3.7 | >95 | [56] |
| Hybrid organo-silica | CTP (air) | 60–22 | 0.3–7.5 | 21–2 | >98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elma, M.; Mujiyanti, D.R.; Ismail, N.M.; Bilad, M.R.; Rahma, A.; Rahman, S.K.; Fitriani, F.; Rakhman, A.; Rampun, E.L.A. Development of Hybrid and Templated Silica-P123 Membranes for Brackish Water Desalination. Polymers 2020, 12, 2644. https://doi.org/10.3390/polym12112644
Elma M, Mujiyanti DR, Ismail NM, Bilad MR, Rahma A, Rahman SK, Fitriani F, Rakhman A, Rampun ELA. Development of Hybrid and Templated Silica-P123 Membranes for Brackish Water Desalination. Polymers. 2020; 12(11):2644. https://doi.org/10.3390/polym12112644
Chicago/Turabian StyleElma, Muthia, Dwi Rasy Mujiyanti, Noor Maizura Ismail, Muhammad Roil Bilad, Aulia Rahma, Sazila Karina Rahman, Fitriani Fitriani, Arief Rakhman, and Erdina Lulu Atika Rampun. 2020. "Development of Hybrid and Templated Silica-P123 Membranes for Brackish Water Desalination" Polymers 12, no. 11: 2644. https://doi.org/10.3390/polym12112644