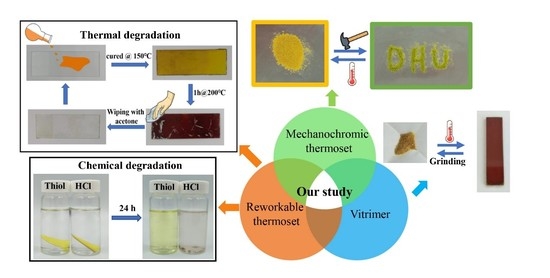
Reprocessable, Reworkable, and Mechanochromic Polyhexahydrotriazine Thermoset with Multiple Stimulus Responsiveness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PHT
2.3. Reprocessing of PHT
2.4. Chemical Reworkability
2.5. Thermal Reworkability
2.6. Characterizations
3. Results
3.1. Preparation of PHT
3.2. Reprocessing and Healing of PHT
3.3. Thermal Stress Relaxation
3.4. Reworkability and Solvent Resistance
3.5. Mechanochromism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling Chemical advances are increasing the proportion of polymer waste that can be recycled. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wong, C.P. Syntheses and characterizations of thermally reworkable epoxy resins. Part I. J. Polym. Sci. A Polym. Chem. 1999, 37, 2991–3001. [Google Scholar] [CrossRef]
- Chen, X.X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.B.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Edit. 2019, 58, 9682–9695. [Google Scholar] [CrossRef]
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.H.; Qi, H.J.; Zhang, W. Heat- or Water-Driven Malleability in a Highly Recyclable Covalent Network Polymer. Adv. Mater. 2014, 26, 3938–3942. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakma, P.; Digby, Z.A.; Via, J.; Shulman, M.P.; Sparks, J.L.; Konkolewicz, D. Tuning thermoresponsive network materials through macromolecular architecture and dynamic thiol-Michael chemistry. Polym. Chem. 2018, 9, 4744–4756. [Google Scholar] [CrossRef]
- De Alwis Watuthanthrige, N.; Ahammed, B.; Dolan, M.T.; Fang, Q.; Wu, J.; Sparks, J.L.; Zanjani, M.B.; Konkolewicz, D.; Ye, Z. Accelerating dynamic exchange and self-healing using mechanical forces in crosslinked polymers. Mater. Horiz. 2020, 7, 1581–1587. [Google Scholar] [CrossRef]
- Ishibashi, J.S.A.; Kalow, J.A. Vitrimeric Silicone Elastomers Enabled by Dynamic Meldrum’s Acid-Derived Cross-Links. ACS Macro Lett. 2018, 7, 482–486. [Google Scholar] [CrossRef]
- Chakma, P.; Morley, C.N.; Sparks, J.L.; Konkolewicz, D. Exploring How Vitrimer-like Properties Can Be Achieved from Dissociative Exchange in Anilinium Salts. Macromolecules 2020, 53, 1233–1244. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; De Hoe, G.X.; Snyder, R.L.; Dichtel, W.R.; Hillmyer, M.A. Approaches to Sustainable and Continually Recyclable Cross-Linked Polymers. ACS Sustain. Chem. Eng. 2018, 6, 11145–11159. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Zhou, Y.; Wang, J.; Wu, Z.; Wang, H.; Chen, H. Self-Healing of Polarizing Films via the Synergy between Gold Nanorods and Vitrimer. Adv. Mater. 2019, 31, e1900363. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Wu, Y.; Zhao, X.; Zhang, Y. Rapid Stress Relaxation and Moderate Temperature of Malleability Enabled by the Synergy of Disulfide Metathesis and Carboxylate Transesterification in Epoxy Vitrimers. ACS Macro Lett. 2019, 8, 255–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Liang, G.; Gu, A. Developing Reversible Self-Healing and Malleable Epoxy Resins with High Performance and Fast Recycling through Building Cross-Linked Network with New Disulfide-Containing Hardener. Ind. Eng. Chem. Res. 2018, 57, 12397–12406. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.; Chen, J.; Xiong, J.; Wang, D.; Wang, S.; Wu, S.; Guo, B. Tuning the mechanical and dynamic properties of imine bond crosslinked elastomeric vitrimers by manipulating the crosslinking degree. Polym. Chem. 2020, 11, 1348–1355. [Google Scholar] [CrossRef]
- Memon, H.; Liu, H.Y.; Rashid, M.A.; Chen, L.; Jiang, Q.R.; Zhang, L.Y.; Wei, Y.; Liu, W.S.; Qiu, Y.P. Vanillin-Based Epoxy Vitrimer with High Performance and Closed-Loop Recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, N.; Adraro, Y.A.; Wang, B.; Liu, Y.; Yuan, W.; Xu, X.; Huang, Y.; Hu, Z. Imine or Secondary Amine-Derived Degradable Polyaminal: Low-Cost Matrix Resin with High Performance. ACS Sustain. Chem. Eng. 2020, 8, 1943–1953. [Google Scholar] [CrossRef]
- Hao, C.; Liu, T.; Zhang, S.; Liu, W.; Shan, Y.; Zhang, J. Triethanolamine-Mediated Covalent Adaptable Epoxy Network: Excellent Mechanical Properties, Fast Repairing, and Easy Recycling. Macromolecules 2020, 53, 3110–3118. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, B.; Zhang, J. Recent development of repairable, malleable and recyclable thermosetting polymers through dynamic transesterification. Polymer 2020, 194, 122392. [Google Scholar] [CrossRef]
- Wu, J.; Yu, X.; Zhang, H.; Guo, J.; Hu, J.; Li, M.-H. Fully Biobased Vitrimers from Glycyrrhizic Acid and Soybean Oil for Self-Healing, Shape Memory, Weldable, and Recyclable Materials. ACS Sustain. Chem. Eng. 2020, 8, 6479–6487. [Google Scholar] [CrossRef]
- Lu, Y.X.; Guan, Z. Olefin metathesis for effective polymer healing via dynamic exchange of strong carbon-carbon double bonds. J. Am. Chem Soc. 2012, 134, 14226–14231. [Google Scholar] [CrossRef]
- Lu, Y.X.; Tournilhac, F.; Leibler, L.; Guan, Z. Making insoluble polymer networks malleable via olefin metathesis. J. Am. Chem Soc. 2012, 134, 8424–8427. [Google Scholar] [CrossRef]
- Tretbar, C.A.; Neal, J.A.; Guan, Z. Direct Silyl Ether Metathesis for Vitrimers with Exceptional Thermal Stability. J. Am. Chem Soc. 2019, 141, 16595–16599. [Google Scholar] [CrossRef]
- Zych, A.; Pinalli, R.; Soliman, M.; Vachon, J.; Dalcanale, E. Polyethylene vitrimers via silyl ether exchange reaction. Polymer 2020, 199, 122567. [Google Scholar] [CrossRef]
- Christensen, P.R.; Scheuermann, A.M.; Loeffler, K.E.; Helms, B.A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem 2019, 11, 442–448. [Google Scholar] [CrossRef]
- He, C.; Christensen, P.R.; Seguin, T.J.; Dailing, E.A.; Wood, B.M.; Walde, R.K.; Persson, K.A.; Russell, T.P.; Helms, B.A. Conformational Entropy as a Means to Control the Behavior of Poly(diketoenamine) Vitrimers In and Out of Equilibrium. Angew. Chem. Int. Ed. Engl. 2020, 59, 735–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffy, F.; Nicolaÿ, R. Transformation of polyethylene into a vitrimer by nitroxide radical coupling of a bis-dioxaborolane. Polym. Chem. 2019, 10, 3107–3115. [Google Scholar] [CrossRef]
- Ricarte, R.G.; Tournilhac, F.; Cloître, M.; Leibler, L. Linear Viscoelasticity and Flow of Self-Assembled Vitrimers: The Case of a Polyethylene/Dioxaborolane System. Macromolecules 2020, 53, 1852–1866. [Google Scholar] [CrossRef]
- Chen, Q.; Qian, X.; Xu, Y.; Yang, Y.; Wei, Y.; Ji, Y. Harnessing the Day-Night Rhythm of Humidity and Sunlight into Mechanical Work Using Recyclable and Reprogrammable Soft Actuators. ACS Appl. Mater. Interfaces 2019, 11, 29290–29297. [Google Scholar] [CrossRef]
- Miao, J.T.; Ge, M.; Peng, S.; Zhong, J.; Li, Y.; Weng, Z.; Wu, L.; Zheng, L. Dynamic Imine Bond-Based Shape Memory Polymers with Permanent Shape Reconfigurability for 4D Printing. ACS Appl. Mater. Interfaces 2019, 11, 40642–40651. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, J.; Zuo, H.; Ning, N.; Zhang, L.; Yu, B.; Tian, M. Photothermal-Induced Self-Healable and Reconfigurable Shape Memory Bio-Based Elastomer with Recyclable Ability. ACS Appl Mater. Interfaces 2019, 11, 1469–1479. [Google Scholar] [CrossRef]
- Ji, F.; Liu, X.; Sheng, D.; Yang, Y. Epoxy-vitrimer composites based on exchangeable aromatic disulfide bonds: Reprocessibility, adhesive, multi-shape memory effect. Polymer 2020, 197, 122514. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Surface engineering of nanosilica for vitrimer composites. Compos. Sci. Technol. 2018, 154, 18–27. [Google Scholar] [CrossRef]
- Garcia, J.M.; Jones, G.O.; Virwani, K.; McCloskey, B.D.; Boday, D.J.; ter Huurne, G.M.; Horn, H.W.; Coady, D.J.; Bintaleb, A.M.; Alabdulrahman, A.M.S.; et al. Recyclable, Strong Thermosets and Organogels via Paraformaldehyde Condensation with Diamines. Science 2014, 344, 732–735. [Google Scholar] [CrossRef]
- Kaminker, R.; Callaway, E.B.; Dolinski, N.D.; Barbon, S.M.; Shibata, M.; Wang, H.; Hu, J.; Hawker, C.J. Solvent-Free Synthesis of High-Performance Polyhexahydrotriazine (PHT) Thermosets. Chem. Mater. 2018, 30, 8352–8358. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Yan, S.; Zhao, J.; Liu, S.; Zhang, M.; Zheng, X.; Jia, L. Multiply fully recyclable carbon fibre reinforced heat-resistant covalent thermosetting advanced composites. Nat. Commun. 2017, 8, 14657. [Google Scholar] [CrossRef] [PubMed]
- Matxain, J.M.; Asua, J.M.; Ruiperez, F. Design of new disulfide-based organic compounds for the improvement of self-healing materials. Phys. Chem. Chem. Phys. 2016, 18, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, S.; Yan, S.; Zhu, J. Readily recyclable carbon fiber reinforced composites based on degradable thermosets: A review. Green Chem. 2019, 21, 5781–5796. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kamada, J.; Koynov, K.; Mohin, J.; Nicolay, R.; Zhang, Y.Z.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Self-Healing Polymer Films Based on Thiol-Disulfide Exchange Reactions and Self-Healing Kinetics Measured Using Atomic Force Microscopy. Macromolecules 2012, 45, 142–149. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. Reversible redox cleavage/coupling of polystyrene with disulfide or thiol groups prepared by atom transfer radical polymerization. Macromolecules 2002, 35, 9009–9014. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; de Luzuriaga, A.R.; Cabanero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz.s 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohishi, T.; Goseki, R.; Otsuka, H. Degradable epoxy resins prepared from diepoxide monomer with dynamic covalent disulfide linkage. Polymer 2016, 82, 319–326. [Google Scholar] [CrossRef]
- Denes, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl Radicals in Organic Synthesis. Chem Rev. 2014, 114, 2587–2693. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Matxain, J.M.; Ruipérez, F.; Martin, R.; Asua, J.M.; Cabañero, G.; Odriozola, I. Transient mechanochromism in epoxy vitrimer composites containing aromatic disulfide crosslinks. J. Mater. Chem. C 2016, 4, 6220–6223. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Lei, H.; Wang, S.; Liaw, D.J.; Cheng, Y.; Yang, X.; Tan, J.; Chen, X.; Gu, J.; Zhang, Y. Tunable and Processable Shape-Memory Materials Based on Solvent-Free, Catalyst-Free Polycondensation between Formaldehyde and Diamine at Room Temperature. ACS Macro Lett. 2019, 8, 582–587. [Google Scholar] [CrossRef]
- Yang, Z.H.; Duan, C.J.; Sun, Y.; Wang, T.M.; Zhang, X.R.; Wang, Q.H. Utilizing Polyhexahydrotriazine (PHT) to Cross-Link Polyimide Oligomers for High-Temperature Shape Memory Polymer. Ind. Eng. Chem. Res. 2019, 58, 10599–10608. [Google Scholar] [CrossRef]
- Chen, Q.M.; Pei, Z.Q.; Xu, Y.S.; Li, Z.; Yang, Y.; Wei, Y.; Ji, Y. A durable monolithic polymer foam for efficient solar steam generation. Chem. Sci. 2018, 9, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.; Ma, S.; Dai, J.; Jia, Z.; Liu, X.; Zhu, J. Hexahydro-s-triazine: A Trial for Acid-Degradable Epoxy Resins with High Performance. ACS Sustain. Chem. Eng. 2017, 5, 4683–4689. [Google Scholar] [CrossRef]
- Zhou, F.; Guo, Z.; Wang, W.; Lei, X.; Zhang, B.; Zhang, H.; Zhang, Q. Preparation of self-healing, recyclable epoxy resins and low-electrical resistance composites based on double-disulfide bond exchange. Compos. Sci. Technol. 2018, 167, 79–85. [Google Scholar] [CrossRef]
- Azcune, I.; Odriozola, I. Aromatic disulfide crosslinks in polymer systems: Self-healing, reprocessability, recyclability and more. Eur. Polym. J. 2016, 84, 147–160. [Google Scholar] [CrossRef]
- Zhang, L.; Rowan, S.J. Effect of Sterics and Degree of Cross-Linking on the Mechanical Properties of Dynamic Poly(alkylurea–urethane) Networks. Macromolecules 2017, 50, 5051–5060. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and Recycling of Highly Cross-Linked Ion-Conducting Networks through Transalkylation Exchanges of C-N Bonds. J. Am. Chem Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. Acs Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Dyre, J.C. Colloquium: The glass transition and elastic models of glass-forming liquids. Rev. Mod. Phys. 2006, 78, 953–972. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Abu-Omar, M.M. Recyclable and Malleable Epoxy Thermoset Bearing Aromatic Imine Bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Yang, S.; Chen, J.S.; Korner, H.; Breiner, T.; Ober, C.K.; Poliks, M.D. Reworkable epoxies: Thermosets with thermally cleavable groups for controlled network breakdown. Chem. Mater. 1998, 10, 1475–1482. [Google Scholar] [CrossRef]
- Johnson, L.M.; Ledet, E.; Huffman, N.D.; Swarner, S.L.; Shepherd, S.D.; Durham, P.G.; Rothrock, G.D. Controlled degradation of disulfide-based epoxy thermosets for extreme environments. Polymer 2015, 64, 84–92. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, H.Y.; Wong, C.P. Syntheses and characterizations of thermally reworkable epoxy resins II. J. Polym Sci. A Polym. Chem. 2000, 38, 3771–3782. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhu, S.; Toendepi, I.; Jiang, Q.; Wei, Y.; Qiu, Y.; Liu, W. Reprocessable, Reworkable, and Mechanochromic Polyhexahydrotriazine Thermoset with Multiple Stimulus Responsiveness. Polymers 2020, 12, 2375. https://doi.org/10.3390/polym12102375
Chen L, Zhu S, Toendepi I, Jiang Q, Wei Y, Qiu Y, Liu W. Reprocessable, Reworkable, and Mechanochromic Polyhexahydrotriazine Thermoset with Multiple Stimulus Responsiveness. Polymers. 2020; 12(10):2375. https://doi.org/10.3390/polym12102375
Chicago/Turabian StyleChen, Li, Siyao Zhu, Innocent Toendepi, Qiuran Jiang, Yi Wei, Yiping Qiu, and Wanshuang Liu. 2020. "Reprocessable, Reworkable, and Mechanochromic Polyhexahydrotriazine Thermoset with Multiple Stimulus Responsiveness" Polymers 12, no. 10: 2375. https://doi.org/10.3390/polym12102375