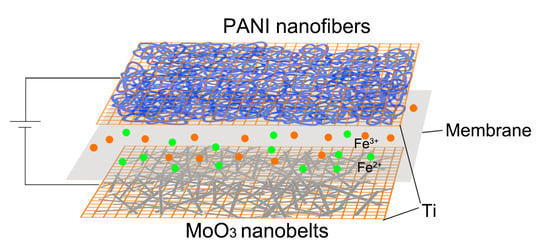
Electrodeposited Polyaniline Nanofibers and MoO3 Nanobelts for High-Performance Asymmetric Supercapacitor with Redox Active Electrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PANI/Ti Electrode
2.3. Synthesis of MoO3 Electrode
2.4. Electrochemical Measurements
2.5. Characterization
3. Results and Discussion
3.1. Morphology of PANI/Ti and MoO3
3.2. Electrochemical Performance
- ; (a) (oxidation reaction)
- ; (b) (reduction reaction)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Piñero, E.; Frackowiak, E.; Béguin, F. High-voltage asymmetric supercapacitors operating in aqueous electrolyte. Appl. Phys. A Mater. Sci. Process. 2006, 82, 567–573. [Google Scholar] [CrossRef]
- Zheng, C.; Yoshio, M.; Qi, L.; Wang, H. A 4 V-electrochemical capacitor using electrode and electrolyte materials free of metals. J. Power Sources 2014, 260, 19–26. [Google Scholar] [CrossRef]
- Mo, Y.; Meng, W.; Xia, Y.; Du, X.; Lin, Z.; Li, W. Facile flame deposit of CNFs/Fe2O3 coating on 304 stainless steel mesh and their high capacitive performance. Electrochim. Acta 2020, 335, 135527. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Yang, H.; Sun, Y.; Hu, C.; Huang, Y. Flexible asymmetric micro-supercapacitors based on Bi2O3 and MnO2 nanoflowers: Larger areal mass promises higher energy density. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Xia, C.; Leng, M.; Tao, W.; Wang, Q.; Gao, Y.; Zhang, Q. Polyaniline/carbon nanotube core–shell hybrid and redox active electrolyte for high-performance flexible supercapacitor. J. Mater. Sci. Mater. Electron. 2019, 30, 4427–4436. [Google Scholar] [CrossRef]
- Kong, L.B.; Zhang, J.; An, J.J.; Luo, Y.C.; Kang, L. MWNTs/PANI composite materials prepared by in-situ chemical oxidative polymerization for supercapacitor electrode. J. Mater. Sci. 2008, 43, 3664–3669. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.L.; Zhao, S.X.; Yu, L.; Zheng, X.X.; Wang, Y.F.; Yu, L.Q.; Nan, C.W.; Cao, G. Oxygen vacancy-enriched MoO3−x nanobelts for asymmetric supercapacitors with excellent room/low temperature performance. J. Mater. Chem. A 2019, 7, 13205–13214. [Google Scholar] [CrossRef]
- Du, P.; Wei, W.; Liu, D.; Kang, H.; Liu, C.; Liu, P. Fabrication of hierarchical MoO3–PPy core–shell nanobelts and “worm-like” MWNTs–MnO2 core–shell materials for high-performance asymmetric supercapacitor. J. Mater. Sci. 2018, 53, 5255–5269. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, Z.Y.; Li, L.; Zhang, Y.; Wei, Y.L.; Wang, L.F.; Zhu, M.F. Polyaniline/multi-walled carbon nanotube composites with core-shell structures as supercapacitor electrode materials. Electrochim. Acta 2010, 55, 3904–3908. [Google Scholar] [CrossRef]
- Xie, H.J.; Gélinas, B.; Rochefort, D. Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. Commun. 2016, 66, 42–45. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Z.; Qian, M.; Lin, T.; Huang, F. A bridge between battery and supercapacitor for power/energy gap by using dual redox-active ions electrolyte. Chem. Eng. J. 2019, 375, 122054. [Google Scholar] [CrossRef]
- Tu, Q.M.; Fan, L.Q.; Pan, F.; Huang, J.L.; Gu, Y.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Wu, J.H. Design of a novel redox-active gel polymer electrolyte with a dual-role ionic liquid for flexible supercapacitors. Electrochim. Acta 2018, 268, 562–568. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Lee, Y.S.; Melo, J.S. Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte. J. Mater. Chem. A 2013, 1, 1086–1095. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.H. High capacitive property for supercapacitor using Fe3+/Fe2+ redox couple additive electrolyte. Electrochim. Acta 2017, 231, 705–712. [Google Scholar] [CrossRef]
- Mo, Y.; Meng, W.; Xia, Y.; Du, X. Redox-active gel electrolyte combined with branched polyaniline nanofibers doped with ferrous ions for ultra-high-performance flexible supercapacitors. Polymers 2019, 11, 1357. [Google Scholar] [CrossRef] [Green Version]
- Chodankar, N.R.; Dubal, D.P.; Lokhande, A.C.; Patil, A.M.; Kim, J.H.; Lokhande, C.D. An innovative concept of use of redox-active electrolyte in asymmetric capacitor based on MWCNTs/MnO2 and Fe2O3 thin films. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Sun, J.; Wu, C.; Sun, X.; Hu, H.; Zhi, C.; Hou, L.; Yuan, C. Recent progresses in high-energy-density all pseudocapacitive-electrode-materials-based asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 9443–9464. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Han, M.K.; Hwang, M.J.; Won, D.H.; Kim, Y.S.; Song, H.J.; Park, Y.J. Massive transformation in titanium-silver alloys and its effect on their mechanical properties and corrosion behavior. Materials 2014, 7, 6194–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, M.B.; Keshmiri, S.H.; Yu, J.; Sadek, A.Z.; Al-Mashat, L.; Moafi, A.; Latham, K.; Li, Y.X.; Wlodarski, W.; Kalantar-zadeh, K. Gas sensing properties of thermally evaporated lamellar MoO3. Sens. Actuators B Chem. 2010, 145, 13–19. [Google Scholar] [CrossRef]
- Rohom, A.B.; Londhe, P.U.; Chaure, N.B. Enhancement of Optical Absorption by Incorporation of Plasmonic Nanoparticles in PANI Films. Nanosci. Nanotechnol. 2016, 6, 83–87. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Humpolíček, P.; Kašpárková, V.; Sapurina, I.; Shishov, M.A.; Varga, M. Conducting Polymers: Polyaniline. In Encyclopedia of Polymer Science and Technology; Wiley: New York, NY, USA, 2015; pp. 1–44. [Google Scholar]
- Windom, B.C.; Sawyer, W.G.; Hahn, D.W. A raman spectroscopic study of MoS2 and MoO3: Applications to tribological systems. Tribol. Lett. 2011, 42, 301–310. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.; Hu, Z.; Wang, J.; Jiao, S. Electrochemical self-assembly of nano-polyaniline film by forced convection and its capacitive performance. RSC Adv. 2017, 7, 3879–3887. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Du, Y.; Feng, Q.; Wang, Z.; Wang, C. Facile method to prepare Pd/graphene-polyaniline nanocomposite and used as new electrode material for electrochemical sensing. J. Mol. Catal. A Chem. 2012, 353–354, 80–86. [Google Scholar] [CrossRef]
- Shim, Y.; Won, M.; Park, S. Electrochemistry of Conductive Polymers VIII: In Situ Spectroelectrochemical Studies of Polyaniline Growth Mechanisms. J. Electrochem. Soc. 2019, 137, 538–544. [Google Scholar] [CrossRef]
- Vol’fkovich, Y.M.; Serdyuk, T.M. Electrochemical capacitors. Russ. J. Electrochem. 2002, 38, 935–959. [Google Scholar] [CrossRef]
- Szkoda, M.; Trzciński, K.; Łapiński, M.; Lisowska-Oleksiak, A. Photoinduced K+ Intercalation into MoO3/FTO Photoanode—The Impact on the Photoelectrochemical Performance. Electrocatalysis 2020, 11, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Li, Q.; Bi, Y.; Cai, M.; Dunn, B.; Glossmann, T.; Liu, J.; Osaka, T.; Sugiura, R.; Wu, B.; et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 2020. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Tian, S.; Li, L.; Yue, Y.; Wu, Y.; Zhu, K. Aqueous supercapacitors of high energy density based on MoO3 nanoplates as anode material. Chem. Commun. 2011, 47, 10058–10060. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ma, G.; Mu, J.; Sun, K.; Lei, Z. Low-cost and high energy density asymmetric supercapacitors based on polyaniline nanotubes and MoO3 nanobelts. J. Mater. Chem. A 2014, 2, 10384–10388. [Google Scholar] [CrossRef]
- Zou, B.X.; Liang, Y.; Liu, X.X.; Diamond, D.; Lau, K.T. Electrodeposition and pseudocapacitive properties of tungsten oxide/polyaniline composite. J. Power Sources 2011, 196, 4842–4848. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Jin, M.; Yao, F.; Kim, T.H.; Le, V.T.; Yue, H.; Gunes, F.; Li, B.; Ghosh, A.; Xie, S.; et al. Asymmetric supercapacitors based on graphene/MnO2 nanospheres and graphene/MoO3 nanosheets with high energy density. Adv. Funct. Mater. 2013, 23, 5074–5083. [Google Scholar] [CrossRef]





| Sample | Electrolyte | Capacitance/F g−1 (Current Density) | Energy Density (Wh/kg) | Power Density (W/kg) | Testing Configuration | Ref. |
|---|---|---|---|---|---|---|
| MoO3 | H2SO4 | 1243 (2 mV/s) | - | - | half-cell | present |
| MoO3 | Li2SO4 | 280 (1 mV/s) | - | - | half-cell | [34] |
| MoO3−x | H2SO4/EG | 1230 (5 A/g) | - | - | half-cell | [10] |
| MoO3 | H2SO4 | 560 (1 A/g) | - | - | half-cell | [35] |
| AC//MoO3−x | H2SO4/EG | 313 (1 A/g) | 111 | 803 | full-cell | [10] |
| AC//MoO3 | Li2SO4 | 30 (2 A/g) | 45 | 450 | full-cell | [34] |
| CNTs/MnO2//MoO3/PPy | Na2SO4 | ~47 (0.24 A/g) | 21 | 220 | full-cell | [11] |
| PANI//MoO3/PANI | H2SO4 | 49 (5 mV/s) | 10 | 53 | full-cell | [36] |
| PANI//MoO3 | H2SO4 | 518 (0.5 A/g) | 72 | 254 | full-cell | [35] |
| GrMnO2//GrMoO3 | Na2SO4 | ~90 (1 A/g) | 43 | 276 | full-cell | [37] |
| PANI//MoO3 | H2SO4/Fe2+/3+ | 197 (1 A/g) | 54 | 900 | full-cell | present |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, W.; Xia, Y.; Ma, C.; Du, X. Electrodeposited Polyaniline Nanofibers and MoO3 Nanobelts for High-Performance Asymmetric Supercapacitor with Redox Active Electrolyte. Polymers 2020, 12, 2303. https://doi.org/10.3390/polym12102303
Meng W, Xia Y, Ma C, Du X. Electrodeposited Polyaniline Nanofibers and MoO3 Nanobelts for High-Performance Asymmetric Supercapacitor with Redox Active Electrolyte. Polymers. 2020; 12(10):2303. https://doi.org/10.3390/polym12102303
Chicago/Turabian StyleMeng, Wei, Yanlin Xia, Chuanguo Ma, and Xusheng Du. 2020. "Electrodeposited Polyaniline Nanofibers and MoO3 Nanobelts for High-Performance Asymmetric Supercapacitor with Redox Active Electrolyte" Polymers 12, no. 10: 2303. https://doi.org/10.3390/polym12102303