Study on the Synthesis and Thermal Stability of Silicone Resin Containing Trifluorovinyl Ether Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Characterization
2.3. Synthesis of the Intermediates and the Silane Monomer
2.3.1. Synthesis of 4-[2-Bromotetrafluoroethoxy]bromobenzene (M1)
2.3.2. Synthesis of 4-[Trifluorovinyl(oxygen)]bromobenzene (M2)
2.3.3. Synthesis of {4-[Trifluorovinyl(oxygen)]phenyl}methyldiethoxysilane (M3)
2.4. Synthesis of the Silicone Resins
2.5. Curing of the Silicone Resins
3. Results and Discussion
3.1. Characterization of the F-SRs
3.2. Curing Behavior of the F-SRs
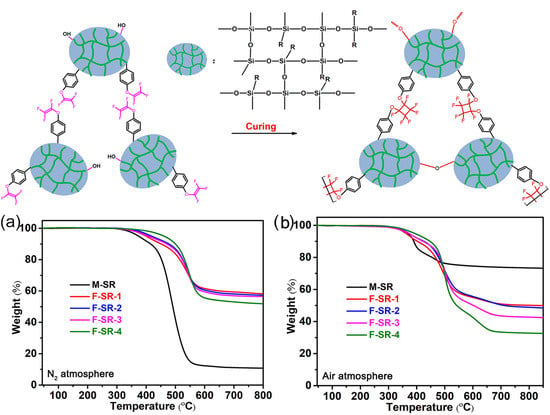
3.3. Thermal Properties and Degradation of F-SRs
3.4. Hydrophobicity of the F-SRs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, C.; Wilkes, G.L. Silicone/Amine Resin Hybrid Materials as Abrasion Resistant Coatings. Chem. Mater. 2001, 13, 3663–3668. [Google Scholar] [CrossRef]
- Zhan, X.; Liu, H.; Zhang, J.; Cheng, J.; Lin, X. Comparative Study of Silicone Resin Cured with a Linear and a Branched Cross-Linking Agent. Ind. Eng. Chem. Res. 2014, 53, 4254–4262. [Google Scholar] [CrossRef]
- Kuo, C.-F.J.; Chen, J.-B. Study on the synthesis and application of silicone resin containing phenyl group. J. Sol.-Gel Sci. Technol. 2015, 76, 66–73. [Google Scholar] [CrossRef]
- Kuo, C.-F.J.; Chen, J.-B.; Shih, C.-Y.; Huang, C.-Y. Silicone resin synthesized by tetraethoxysilane and chlorotrimethylsilane through hydrolysis-condensation reaction. J. Appl. Polym. Sci. 2013, 131, 8. [Google Scholar] [CrossRef]
- Indulekha, K.; Monisha, S.; Thomas, D.; Rajeev, R.; Mathew, D.; Ninan, K.; Cheruvally, G. Polycyclic siloxanes: Base resins for novel high temperature resistant platinum curing transparent silicone adhesives. Int. J. Adhes. Adhes. 2018, 82, 254–262. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Z.; Fan, J.; Qian, C.; Deng, Z.; Gui, D. High Temperature Performance Evaluation and Life Prediction for Titanium Modified Silicone Used in Light-Emitting Diodes Chip Scale Packages. J. Electron. Packag. 2020, 142, 142. [Google Scholar] [CrossRef]
- Gao, J.; Li, Z.; Li, J.; Liu, Y. Ablation Behavior of Silicone Rubber-Benzoxazine-Based Composites for Ultra-High Temperature Applications. Polymers 2019, 11, 1844. [Google Scholar] [CrossRef] [Green Version]
- Coons, J.E.; McKay, M.; Hamada, M. A Bayesian analysis of the compression set and stress–strain behavior in a thermally aged silicone foam. Polym. Degrad. Stab. 2006, 91, 1824–1836. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, H.; Guo, X.; Lu, J. Thermal degradation behaviors of some branched and linear polysiloxanes. Polym. Degrad. Stab. 2006, 91, 1471–1475. [Google Scholar] [CrossRef]
- Wu, C.; Huang, M.; Luo, D.; Jiang, Y.; Yan, M. SiO2 nanoparticles enhanced silicone resin as the matrix for Fe soft magnetic composites with improved magnetic, mechanical and thermal properties. J. Alloy. Compd. 2018, 741, 35–43. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Shen, Y.; Zhou, Y.; Wang, D.; Lei, Z.; Feng, W.; Min, Z. Silicone-based alumina composites synthesized through in situ polymerization for high thermal conductivity and thermal stability. Mater. Lett. 2020, 261, 127002. [Google Scholar] [CrossRef]
- Yang, Z.; Han, S.; Zhang, R.; Feng, S.; Zhang, C.; Zhang, S. Effects of silphenylene units on the thermal stability of silicone resins. Polym. Degrad. Stab. 2011, 96, 2145–2151. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, J.; Wu, Y.; Yu, J.; Yu, L. Synthesis and thermal stability properties of boron-doped silicone resin. J. Appl. Polym. Sci. 2014, 131, 8. [Google Scholar] [CrossRef]
- Grassie, N.; Francey, K.; Macfarlane, I. The thermal degradation of polysiloxanes—Part 4: Poly(dimethyl/diphenyl siloxane). Polym. Degrad. Stab. 1980, 2, 67–83. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Shi, L.; Qi, S.; Cheng, J.; Jin, R. Improvement of thermal resistance of polydimethylsiloxanes with polymethylmethoxysiloxane as crosslinker. Polym. Degrad. Stab. 2008, 93, 242–251. [Google Scholar] [CrossRef]
- Xu, Y.; Long, J.; Zhang, R.; Du, Y.; Guan, S.; Wang, Y.; Huang, L.; Wei, H.; Liu, L.; Huang, Y. Greatly improving thermal stability of silicone resins by modification with POSS. Polym. Degrad. Stab. 2020, 174, 109082. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Liu, L. Effects of TriSilanolIsobutyl-POSS on thermal stability of methylsilicone resin. Polym. Degrad. Stab. 2006, 91, 2731–2738. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zheng, J.; Guo, H.; Guan, X.-X.; Lu, M.; Wu, K.; Liang, L. Synthesis and characterization of vinyl-polyhedral oligomeric silsesquioxanes-reinforced silicone resin with three-dimensional cross-linking structure. J. Appl. Polym. Sci. 2015, 132, 8. [Google Scholar] [CrossRef]
- Rizzo, J.; Harris, F. Synthesis and thermal properties of fluorosilicones containing perfluorocyclobutane rings. Polymer 2000, 41, 5125–5136. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, Q.; Wang, J.; Sun, J.; Jin, K. Post-functionalization of novolac resins by introducing thermo-crosslinkable –OCF=CF2 groups as the side chains: A new strategy for production of thermosetting polymers without releasing volatiles. Polym. Chem. 2016, 7, 4313–4316. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, H.-J.; Jang, J.H.; Cho, E.A.; Henkensmeier, D.; Kim, S.-K.; Oh, I.-H.; Hong, S.-A.; Lim, T.-H. Crosslinked monosulfonated poly(arylene ether) using cyclodimerization of trifluorovinyl ether groups for fuel cell applications. Polym. Int. 2011, 60, 685–691. [Google Scholar] [CrossRef]
- Chang, B.-J.; Kim, D.J.; Kim, J.H.; Lee, S.-B.; Joo, H.J. Sulfonated poly(fluorene-co-sulfone)ether membranes containing perfluorocyclobutane groups for fuel cell applications. J. Membr. Sci. 2008, 325, 989–996. [Google Scholar] [CrossRef]
- Jin, J.; Topping, C.M.; Suresh, S.; Foulger, S.; Rice, N.; Mojazza, B.H.; Smith, D.W.; Smith, D.W. Synthesis and characterization of perfluorocyclobutyl (PFCB) polymers containing pendent phenylphosphine oxide. Polymer 2005, 46, 6923–6932. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, J.; Jin, K.; Diao, S.; Sun, J.; Tong, J.; Fang, Q. Postpolymerization of Functional Organosiloxanes: An Efficient Strategy for Preparation of Low-k Material with Enhanced Thermostability and Mechanical Properties. Macromolecules 2014, 47, 6311–6315. [Google Scholar] [CrossRef]
- Wang, J.; Jin, K.; Sun, J.; Fang, Q. Dendrimeric organosiloxane with thermopolymerizable –OCF=CF2 groups as the arms: Synthesis and transformation to the polymer with both ultra-low k and low water uptake. Polym. Chem. 2016, 7, 3378–3382. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.W.; Babb, D.A. Perfluorocyclobutane Aromatic Polyethers. Synthesis and Characterization of New Siloxane-Containing Fluoropolymers. Macromolecules 1996, 29, 852–860. [Google Scholar] [CrossRef]
- Smith, D.W.; Ji, J.; Narayan-Sarathy, S.; Neilson, R.H.; Babb, D.A. Fluorosilicones Containing the Perfluorocyclobutane Aromatic Ether Linkage. ACS Symp. Ser. 2000, 729, 308–321. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, J.; Jin, K.; Luo, Y.; Zhou, J.; Wang, Y.; Sun, J.; Zheng, S.; Fang, Q. A New Four-Arm Organosiloxane with Thermopolymerizable Trifluorovinyl ether Groups: Synthesis and Conversion to the Polymer with both Low Dielectric Constant and Low Water Uptake. Macromol. Chem. Phys. 2017, 218, 1700010. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, X.; Zhang, D.; Liu, Y.; Huang, G. Synthesis and thermal properties of modified room temperature vulcanized (RTV) silicone rubber using polyhedral oligomeric silsesquioxane (POSS) as a cross linking agent. RSC Adv. 2014, 4, 41453–41460. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, L.; Diao, S.; Feng, S.; Zhang, C. Study on the synthesis and thermal degradation of silicone resin containing silphenylene units. Thermochim. Acta 2011, 521, 170–175. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Properties Enhancement of Room Temperature Vulcanized Silicone Rubber by Rosin Modified Aminopropyltriethoxysilane as a Cross-linking Agent. ACS Sustain. Chem. Eng. 2017, 5, 10002–10010. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]













| Sample No. | R/Si | MTES (mmol) | DMDES (mmol) | M3 (mmol) | RF/R | Yield (%) | |
|---|---|---|---|---|---|---|---|
| Designed | Found * | ||||||
| M-SR | 1.5 | 15 | 15 | 0 | 0 | 0 | 81.5 |
| F-SR-1 | 1.5 | 15 | 10 | 5 | 0.11 | 0.10 | 80.4 |
| F-SR-2 | 1.5 | 15 | 7.5 | 7.5 | 0.17 | 0.17 | 82.7 |
| F-SR-3 | 1.5 | 15 | 5 | 10 | 0.22 | 0.23 | 81.1 |
| F-SR-4 | 1.5 | 15 | 0 | 15 | 0.33 | 0.31 | 82.0 |
| Sample No. | Temperature for 5% Mass Loss (°C) | Temperature for 10% Mass Loss (°C) | Temperature for the Maximum Rate of Mass Loss (°C) | Residue at 800 °C (%) |
|---|---|---|---|---|
| M-SR | 374 | 415 | 490 | 10.8 |
| F-SR-1 | 400 | 452 | 537 | 58.3 |
| F-SR-2 | 418 | 478 | 538 | 57.4 |
| F-SR-3 | 409 | 470 | 541 | 56.5 |
| F-SR-4 | 461 | 500 | 547 | 51.9 |
| Sample No. | Temperature for 5% Mass Loss (°C) | Temperature for 10% Mass Loss (°C) | Temperature for Maximum rate of Mass Loss (°C) | Residue at 850 °C (%) |
|---|---|---|---|---|
| M-SR | 362 | 390 | 391 | 73.2 |
| F-SR-1 | 362 | 402 | 493 | 50.0 |
| F-SR-2 | 372 | 425 | 498 | 48.5 |
| F-SR-3 | 370 | 422 | 502 | 42.5 |
| F-SR-4 | 392 | 447 | 499 | 32.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Yao, J.; Mu, Q.; Peng, D.; Zhao, H.; Yang, Z. Study on the Synthesis and Thermal Stability of Silicone Resin Containing Trifluorovinyl Ether Groups. Polymers 2020, 12, 2284. https://doi.org/10.3390/polym12102284
Huang R, Yao J, Mu Q, Peng D, Zhao H, Yang Z. Study on the Synthesis and Thermal Stability of Silicone Resin Containing Trifluorovinyl Ether Groups. Polymers. 2020; 12(10):2284. https://doi.org/10.3390/polym12102284
Chicago/Turabian StyleHuang, Rui, Jinshui Yao, Qiuhong Mu, Dan Peng, Hui Zhao, and Zhizhou Yang. 2020. "Study on the Synthesis and Thermal Stability of Silicone Resin Containing Trifluorovinyl Ether Groups" Polymers 12, no. 10: 2284. https://doi.org/10.3390/polym12102284