Novel Conjugated Polymers Containing 3-(2-Octyldodecyl)thieno[3,2-b]thiophene as a π-Bridge for Organic Photovoltaic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monomer Syntheses
2.2.1. Synthesis of 3-(2-octyldodecyl)thieno[3,2-b]thiophene (1)
2.2.2. Synthesis of tributyl(6-(2-octyldodecyl)thieno[3,2-b]thiophen-2-yl)stannane (2)
2.2.3. Synthesis of 5,6-difluoro-4,7-bis(6-(2-octyldodecyl)thieno[3,2-b]thiophen-2-yl)benzo[c][1,2,5]thiadiazole (3)
2.2.4. Synthesis of 4,7-bis(5-bromo-6-(2-octyldodecyl)thieno[3,2-b]thiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole (ODTTBT)
2.3. Polymerization Procedure
2.3.1. PT-ODTTBT
2.3.2. PTT-ODTTBT
3. Results and Discussion
3.1. Synthesis and Characterization of Polymers
3.2. Optical and Electrochemical Properties
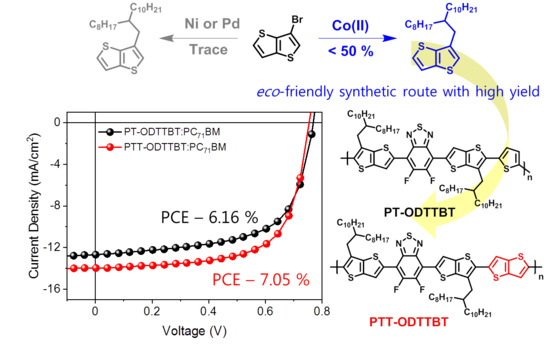
3.3. Photovoltaic Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.-Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yang, Y. Narrowing the Band Gap: The Key to High-Performance Organic Photovoltaics. Accounts Chem. Res. 2020, 53, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photon- 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Kippelen, B.; Brédas, J.-L. Organic photovoltaics. Energy Environ. Sci. 2009, 2, 251–261. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Hong, L.; Zhang, T.; Xu, Y.; Xian, K.; Gao, B.; Qin, J.; Zhang, J.; Wei, Z.; et al. Achieving Over 15% Efficiency in Organic Photovoltaic Cells via Copolymer Design. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.-B.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Chen, Z.; Dong, S.; Zheng, N.; Ying, L.; Jiang, X.-F.; Liu, F.; Huang, F.; Cao, Y. A Novel Naphtho[1,2-c: 5,6-c′]Bis([1,2,5]Thiadiazole)-Based Narrow-Bandgap π-Conjugated Polymer with Power Conversion Efficiency Over 10%. Adv. Mater. 2016, 28, 9811–9818. [Google Scholar] [CrossRef]
- Song, X.; Gasparini, N.; Ye, L.; Yao, H.; Hou, J.; Ade, H.; Baran, D. Controlling Blend Morphology for Ultrahigh Current Density in Nonfullerene Acceptor-Based Organic Solar Cells. ACS Energy Lett. 2018, 3, 669–676. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Zhu, J.; Hou, J. Over 14% Efficiency in Polymer Solar Cells Enabled by a Chlorinated Polymer Donor. Adv. Mater. 2018, 30, e1800868. [Google Scholar] [CrossRef]
- Li, H.; Wu, Q.; Zhou, R.; Shi, Y.; Yang, C.; Zhang, Y.; Zhang, J.; Zou, W.; Deng, D.; Lu, K.; et al. Liquid-Crystalline Small Molecules for Nonfullerene Solar Cells with High Fill Factors and Power Conversion Efficiencies. Adv. Energy Mater. 2018, 9. [Google Scholar] [CrossRef]
- Espinosa, N.; Hösel, M.; Angmo, D.; Krebs, F.C. Solar cells with one-day energy payback for the factories of the future. Energy Environ. Sci. 2012, 5, 5117–5132. [Google Scholar] [CrossRef]
- Kapnopoulos, C.; Mekeridis, E.D.; Tzounis, L.; Polyzoidis, C.; Zachariadis, A.; Tsimikli, S.; Gravalidis, C.; Laskarakis, A.; Vouroutzis, N.; Logothetidi, S. Fully gravure printed organic photovoltaic modules: A straight forward process with a high potential for large scale production. Sol. Energy Mat. Sol. C. 2016, 144, 724–731. [Google Scholar] [CrossRef]
- Krebs, F.C.; Tromholt, T.; Jørgensen, M. Upscaling of polymer solar cell fabrication using full roll-to-roll processing. Nanoscale 2010, 2, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, L.; Gravalidis, C.; Papamichail, A.; Logothetidis, S. Enhancement of P3HT:PCBM Photovoltaic Shells Efficiency Incorporating Core-shell Au@Ag Plasmonic Nanoparticles1. Mater. Today: Proc. 2016, 3, 832–839. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef] [Green Version]
- Peet, J.; Heeger, A.J.; Bazan, G.C. “Plastic” Solar Cells: Self-Assembly of Bulk Heterojunction Nanomaterials by Spontaneous Phase Separation. Acc. Chem. Res. 2009, 42, 1700–1708. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fréchet, J.M.J. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Chen, J.W.; Cao, Y. Development of Novel Conjugated Donor Polymers for High-Efficiency Bulk-Heterojunction Photovoltaic Devices. Acc. Chem. Res. 2009, 42, 1709–1718. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Song, C.E.; Kim, B.; Kang, I.-N.; Shin, W.S.; Hwang, D.-H. Thieno[3,2-b]thiophene-Substituted Benzo[1,2-b:4,5-b′]dithiophene as a Promising Building Block for Low Bandgap Semiconducting Polymers for High-Performance Single and Tandem Organic Photovoltaic Cells. Chem. Mater. 2014, 26, 1234–1242. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.B.; Xu, F.; Kim, D.; Kwak, J.; Grimsdale, A.C.; Hwang, D.-H. Effect of p-conjugated bridges of TPD-based medium bandgap conjugated copolymers for efficient tandem organic photovoltaic cells. Energy Environ. Sci. 2014, 7, 4118–4131. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.B.; Jung, I.H.; Grimsdale, A.C.; Yoon, S.C.; Yang, H.; Hwang, D.-H. Well-controlled thieno[3,4-c]pyrrole-4,6-(5H)-dione based conjugated polymers for high performance organic photovoltaic cells with the power conversion efficiency exceeding 9%. Energy Environ. Sci. 2015, 8, 2352–2356. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Chen, S.; Guo, X.; Zhang, M.; Li, X.; Li, Y.; Wang, H. Effects of π-Conjugated Bridges on Photovoltaic Properties of Donor-π-Acceptor Conjugated Copolymers. Macromolecules 2012, 45, 1208–1216. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, J.-H.; Ahn, J.J.; Hwang, D.-H. Photovoltaic properties of a new quinoxaline-based copolymer with Thieno[3,2-b]thiophene side chain for organic photovoltaic cell applications. Dye. Pigment. 2016, 133, 324–332. [Google Scholar] [CrossRef]
- Cai, D.-J.; Lin, P.-H.; Liu, C.-Y. Cobalt-Catalyzed Reductive Alkylation of Heteroaryl Bromides: One-Pot Access to Alkylthiophenes, -furans, -selenophenes, and -pyrroles. Eur. J. Org. Chem. 2015, 24, 5448–5452. [Google Scholar] [CrossRef]
- Meager, I.; Nikolka, M.; Schroeder, B.C.; Nielsen, C.B.; Planells, M.; Bronstein, H.; Rumer, J.W.; James, D.I.; Ashraf, R.S.; Sadhanala, A.; et al. Thieno[3,2-b]thiophene Flanked Isoindigo Polymers for High Performance Ambipolar OFET Applications. Adv. Funct. Mater. 2014. [Google Scholar] [CrossRef]
- Ni, Z.; Dong, H.; Wang, H.; Ding, S.; Zou, Y.; Zhao, Q.; Zhen, Y.; Liu, F.; Jiang, L.; Hu, W. Quinoline-Flanked Diketopyrrolopyrrole Copolymers Breaking through Electron Mobility over 6 cm2 V−1 s−1 in Flexible Thin Film Devices. Adv. Mater. 2018, 30, 1704843. [Google Scholar] [CrossRef]
- Kini, G.P.; Oh, S.; Abbas, Z.; Rasool, S.; Jahandar, M.; Song, C.E.; Lee, S.K.; Shin, W.S.; So, W.-W.; Lee, J.-C. Effects on Photovoltaic Performance of Dialkyloxy-benzothiadiazole Copolymers by Varying the Thienoacene Donor. ACS Appl. Mater. Interfaces 2017, 9, 12617–12628. [Google Scholar] [CrossRef]
- Jo, J.W.; Jung, J.W.; Wang, H.-W.; Kim, P.; Russell, T.; Jo, W.H. Fluorination of Polythiophene Derivatives for High Performance Organic Photovoltaics. Chem. Mater. 2014, 26, 4214–4220. [Google Scholar] [CrossRef]
- Diao, Y.; Tee, B.C.K.; Giri, G.; Xu, J.; Kim, D.H.; Becerril, H.A.; Stoltenberg, R.M.; Lee, T.H.; Xue, G.; Mannsfeld, S.C.B.; et al. Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains. Nat. Mater. 2013, 12, 665–671. [Google Scholar] [CrossRef] [PubMed]







| Mn (kDa) a | PDIs a | λmax, abs (nm) | λedge (nm) | Egopt (eV) b | EHOMO (eV) c | ELUMO (eV) d | |
|---|---|---|---|---|---|---|---|
| Film | |||||||
| PT-ODTTBT | 82.3 | 1.26 | 437,681 | 752 | 1.65 | -5.48 | -3.83 |
| PTT-ODTTBT | 95.1 | 1.25 | 462,688 | 733 | 1.69 | -5.26 | -3.57 |
| Voc (V) | Jsc (mA/cm2) | Jcalc. (mA/cm2) | FF (%) | PCE (%) | µh (cm2 v−1 s−1) | |
|---|---|---|---|---|---|---|
| PT-ODTTBT :PC71BM a | 0.77 (0.77 ± 0.01) | 12.09 (11.98 ± 0.16) | 11.25 | 63.37 (62.13 ± 0.46) | 5.83 (5.77 ± 0.55) | |
| PT-ODTTBT :PC71BM b | 0.77 (0.77 ± 0.01) | 12.68 (12.24 ± 0.33) | 12.06 | 63.24 (63.15 ± 0.39) | 6.16 (5.97 ± 0.13) | 2.55 × 10–4 |
| PTT-ODTTBT :PC71BM a | 0.75 (0.76 ± 0.01) | 13.13 (12.93 ± 0.18) | 12.75 | 67.88 (68.19 ± 0.38) | 6.70 (6.67 ± 0.04) | |
| PTT-ODTTBT :PC71BM b | 0.75 (0.75 ± 0.01) | 13.96 (13.83 ± 0.11) | 13.32 | 66.94 (66.47 ± 0.63) | 7.05 (6.88 ± 0.12) | 7.08 × 10–3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.-W.; Park, J.B.; Park, H.J.; Hwang, D.-H. Novel Conjugated Polymers Containing 3-(2-Octyldodecyl)thieno[3,2-b]thiophene as a π-Bridge for Organic Photovoltaic Applications. Polymers 2020, 12, 2121. https://doi.org/10.3390/polym12092121
Ha J-W, Park JB, Park HJ, Hwang D-H. Novel Conjugated Polymers Containing 3-(2-Octyldodecyl)thieno[3,2-b]thiophene as a π-Bridge for Organic Photovoltaic Applications. Polymers. 2020; 12(9):2121. https://doi.org/10.3390/polym12092121
Chicago/Turabian StyleHa, Jong-Woon, Jong Baek Park, Hea Jung Park, and Do-Hoon Hwang. 2020. "Novel Conjugated Polymers Containing 3-(2-Octyldodecyl)thieno[3,2-b]thiophene as a π-Bridge for Organic Photovoltaic Applications" Polymers 12, no. 9: 2121. https://doi.org/10.3390/polym12092121