Synthesis and Characterisation of Graphene Oxide-Silica-Chitosan for Eliminating the Pb(II) from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
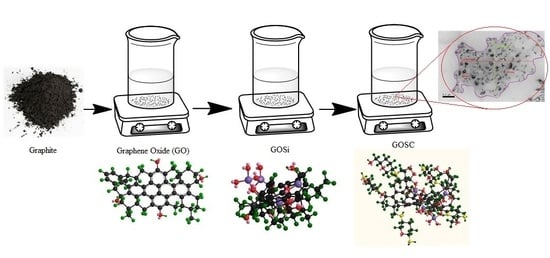
2.2. Preparation of Graphene Oxide-Silica-Chitosan
2.3. Adsorption Process
3. Results and Discussion
3.1. Analysis and Characterisation of Graphene Oxide-Silica-Chitosan Adsorbent
3.1.1. FT-IR Analysis
3.1.2. TEM Analysis
3.1.3. SEM and Mapping Technique
3.1.4. Raman Spectroscopy Analysis
3.1.5. Surface Area Analysis by Brunauer Emmett Teller (BET)
3.2. Batch Experiments of Adsorption
3.2.1. Effect of Chitosan Percentage Ratio
3.2.2. Effect of Adsorbent Dosage
3.2.3. Effect of Initial Concentration
3.2.4. Effect of pH
3.2.5. Effect of Contact Time
3.3. Adsorption Isotherms
3.4. Kinetics Study
3.5. Regeneration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fadzil, F.; Ibrahim, S.; Hanafiah, M.A. Adsorption of lead (II) onto organic acid modified rubber leaf powder: Batch and column studies. Process. Saf. Environ. Prot. 2016, 100, 1–8. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes By Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Bai, R. Adsorptive removal of copper ions with highly porous chitosan/cellulose acetate blend hollow fiber membranes. J. Membr. Sci. 2006, 284, 313–322. [Google Scholar] [CrossRef]
- Azizkhani, S.; Mahmoudi, E.; Abdullah, N.; Ismail, M.H.S.; Mohammad, A.W.; Aslina, S. Removal of Cadmium (II) by Graphene Oxide-Chitosan Adsorbent from Aqueous Solution. Int. J. Eng. Technol. 2018, 7, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Rajput, S.; Pittman, C.U.; Mohan, D. Magnetic Magnetite (Fe3O4) Nanoparticle Synthesis and Applications for Lead (Pb2+) and Chromium (Cr6+) Removal from Water. J. Colloid Interface Sci. 2015, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, N.; Parvini, M.; Niavarani, Z. High surface area and mesoporous graphene/activated carbon composite for adsorption of Pb (II) from wastewater. J. Environ. Chem. Eng. 2015, 3, 2697–2706. [Google Scholar] [CrossRef]
- Xu, Y.; Dang, Q.; Liu, C.; Yan, J.; Fan, B.; Cai, J.; Li, J. Preparation and characterization of carboxyl-functionalized chitosan magnetic microspheres and submicrospheres for Pb2+ removal. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 353–364. [Google Scholar] [CrossRef]
- Huang, X.; Pan, M. The highly efficient adsorption of Pb (II) on graphene oxides: A process combined by batch experiments and modeling techniques. J. Mol. Liq. 2016, 215, 410–416. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Djedidi, Z.; Bouda, M.; Souissi, M.A.; Cheikh RBen Mercier, G.; Tyagi, R.D.; Blais, J.F. Metals removal from soil, fly ash and sewage sludge leachates by precipitation and dewatering properties of the generated sludge. J. Hazard. Mater. 2009, 172, 1372–1382. [Google Scholar] [CrossRef]
- Tan, P.; Sun, J.; Hu, Y.; Fang, Z.; Bi, Q.; Chen, Y.; Cheng, J. Adsorption of Cu2+, Cd2+ and Ni2+ from aqueous single metal solutions on graphene oxide membranes. J. Hazard. Mater. 2015, 297, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zewail, T.M.; Yousef, N.S. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex. Eng. J. 2015, 54, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.; Megeri, G.B.; Thomas, C.; Bhargava, H.; Jeevitha, C.; Chandrashekar, S.; Madhu, G.M. Adsorption and optimization studies of lead from aqueous solution using γ-Alumina. J. Environ. Chem. Eng. 2015, 3, 30–39. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.; Liu, X.; Chen, C.; Li, G.; Meng, Y. Graphene oxides cross-linked with hyperbranched polyethylenimines: Preparation, characterization and their potential as recyclable and highly efficient adsorption materials for lead (II) ions. Chem. Eng. J. 2016, 285, 698–708. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Gong, J.L.; Zeng, G.M.; Ou, X.M.; Chang, Y.N.; Deng, C.H.; Huang, S.Y. Magnetic chitosan-graphene oxide composite for anti-microbial and dye removal applications. Int. J. Biol. Macromol. 2016, 82, 702–710. [Google Scholar] [CrossRef]
- Gao, W. The Chemistry of Graphene Oxide: Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–95. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ang, W.L.; Ng, C.Y.; Ng, L.Y.; Mohammad, A.W.A. Benamor, Distinguishing characteristics and usability of graphene oxide based on different sources of graphite feedstock. J. Colloid Interface Sci. 2019, 542, 429–440. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Kou, L.; Gao, C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Dubey, R.; Bajpai, J.; Bajpai, A.K. Chitosan-alginate nanoparticles (CANPs) as potential nanosorbent for removal of Hg (II) ions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 32–44. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ba-Abbad, M.M.; Mohammad, A.W. Novel nanohybrid polysulfone membrane embedded with silver nanoparticles on graphene oxide nanoplates. Chem. Eng. J. 2015, 277, 1–10. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. Comparison of Pb(II) adsorption onto graphene oxide prepared from natural graphites: Diagramming the Pb(II) adsorption sites. Appl. Surf. Sci. 2016, 364, 620–627. [Google Scholar] [CrossRef]
- Rana, V.K.; Choi, M.C.; Kong, J.Y.; Kim, G.Y.; Kim, M.J.; Kim, S.H.; Ha, C.S. Synthesis and drug-delivery behavior of chitosan-functionalized graphene oxide hybrid nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Ge, H.; Ma, Z. Microwave preparation of triethylenetetramine modified graphene oxide/chitosan composite for adsorption of Cr (VI). Carbohydr. Polym. 2015, 131, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Online, V.A.; Zhang, M.; Zhao, H.; Yang, S.; Arkin, A. Functionalized mesoporous silica material and anionic dye adsorption: MCM-41 incorporated with amine groups for competitive adsorption of Acid Fuchsine and Acid Orange II. RSC Adv. 2014. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P.; Schwan, J.; Ehrhardt, H. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 2013, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Guo, X.; Qu, L.; Tian, M.; Zhu, S.; Zhang, X.; Tang, X.; Sun, K. Chitosan/Graphene Oxide Composite as an Effective Adsorbent for Reactive Red Dye Removal. Water Environ. Res. 2016, 88, 579–588. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Muralidhara, H.B.; Nayaka, Y.A.; Balasubramanyam, J.; Hanumanthappa, H. Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Singh Tomer, N.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization-Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Laus, R.; Gosta, T.G.; Szpoganicz, B.; Fávere, V.T. Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J. Hazard. Mater. 2010, 183, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, X.; Meng, X.; Yu, G.; Wang, D. Removal of Pb(II) from aqueous solution using dithiocarbamate modified chitosan beads with Pb(II) as imprinted ions. Chem. Eng. J. 2013, 220, 412–419. [Google Scholar] [CrossRef]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Hou, S. Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
- Du, Q.; Sun, J.; Li, Y.; Yang, X.; Wang, X.; Wang, Z.; Xia, L. Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem. Eng. J. 2014, 245, 99–106. [Google Scholar] [CrossRef]
- Shahmohammadi-kalalagh, S.; Babazadeh, H.; Manshouri, M. Isotherm and Kinetic Studies on Adsorption of Pb, Zn and Cu by Kaolinite. Casp. J. Environ. Sci. 2011, 9, 243–255. [Google Scholar]
- Liu, J.; Wang, X. Novel Silica-Based Hybrid Adsorbents: Lead (II) Adsorption Isotherms. Sci. J. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Abdulla, F.A. Roof rainwater harvesting systems for household water supply in Jordan. Desalination 2009, 243, 195–207. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Saryel-deen, R.A.; Mostafa, M.K.; Peters, R.W. Artificial Intelligence for Organochlorine Pesticides Removal from Aqueous Solutions Using Entrapped nZVI in Alginate Biopolymer. In Proceedings of the Artificial Intelligence for Organochlorine Pesticides Removal from Aqueous Solutions Using Entrapped Meeting, Minneapolis, MN, USA, 2 November 2017. [Google Scholar]
- Xu, Z.; Cai, J.; Pan, B. Mathematically modeling fixed-bed adsorption in aqueous systems. J. Zhejiang Univ. Sci. A 2013, 14, 155–176. [Google Scholar] [CrossRef] [Green Version]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C. Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.E.; Park, J.H.; Chung, J.W.; Lee, C.Y.; Kang, S. Removal of Pb and Cu ions from aqueous solution by Mn3O4-coated activated carbon. J. Ind. Eng. Chem. 2015, 21, 470–475. [Google Scholar] [CrossRef] [Green Version]













| Adsorbent | SBET (m2/g) | Sext (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) (Average Pore Diameter) |
|---|---|---|---|---|
| GO | 6.33 (+/−2%) | 6.044 (+/−2%) | 1.27 | 61 |
| GOSCh | 10.16 (+/−2%) | 10.16 (+/−2%) | 1.078 | 70.8 |
| 1 g of GO-Si | Initial Concentration (mg/L) | Q% of Pb(II) | Q (mg/g) of Pb(II) |
|---|---|---|---|
| 20% of chitosan | 60 | 83.33 (+/−2%) | 50 (+/−1) |
| 40% of chitosan | 60 | 88.34 (+/−2%) | 53 (+/−1) |
| 60% of chitosan | 60 | 80.00 (+/−2%) | 48 (+/−1) |
| 80% of chitosan | 60 | 75.00 (+/−2%) | 45 (+/−1) |
| Models | Parameters | Pb(II) |
|---|---|---|
| Freundlich | k (mg/g) | 0.85 |
| 1/n | 0.92 | |
| Q max (mg/g) | 19.29 | |
| R2 | 0.99 | |
| MPSD | 0.33 | |
| Langmuir | b (L/mg) | 0.0022 |
| Rl | 0.84 | |
| Q max (mg/g) | 256.41 | |
| R2 | 0.93 | |
| MPSD | 0.63 | |
| Harkins-Jura | B | 1.91 |
| A (L/g) | 68.96 | |
| R2 | 0.749 | |
| MPSD | 6.99 | |
| Duldinin-Redushkevich | B (mol2/kJ2) | 0.00004 |
| Q max (mg/g) | 47.322 | |
| R2 | 0.796 | |
| MPSD | 2.77 | |
| Temkin | A (L/g) | 0.647 |
| b | 94.636 | |
| R2 | 0.94 | |
| MPSD | 3.26 |
| Models | Parameters | Pb(II) |
|---|---|---|
| Pseudo-first-order | K (h−1) | 0.00046 |
| R2 | 0.5774 | |
| Pseudo-second-order | K (gmg−1 h−1) | 0.0134 |
| Q (mg/g) | 32.786 | |
| R2 | 0.999 | |
| MPSD | 0.0033 | |
| Elovich | ἀ (mg/(g min)) | 161.65 |
| β (g/mg) | 0.333 | |
| R2 | 0.8958 | |
| MPSD | 7.52 | |
| Intra-particle | K (mgg−1 h0.5) | 0.9215 |
| Ci | 23.054 | |
| R2 | 0.736 | |
| MPSD | 5.57 |
| Previous Study | Adsorbate | Modifying Method | Maximum Adsorption Capacity |
|---|---|---|---|
| Xu et al 2015 [7] | Pb(II) | Carboxyl-functionalised Chitosan Magnetic Microspheres | 164.81 mg/g |
| Navid Saeidi et al 2015 [6] | Pb(II) | Graphene/Activated Carbon Composite | 217.00 mg/g |
| Lee et al 2015 [47] | Pb(II) | Mn3O4-Coated Activated Carbon | 59.52 mg/g |
| This Study | Pb(II) | Graphene oxide-silica-chitosan | 256.41 mg/g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizkhani, S.; Mahmoudi, E.; Abdullah, N.; Ismail, M.H.S.; Mohammad, A.W.; Hussain, S.A. Synthesis and Characterisation of Graphene Oxide-Silica-Chitosan for Eliminating the Pb(II) from Aqueous Solution. Polymers 2020, 12, 1922. https://doi.org/10.3390/polym12091922
Azizkhani S, Mahmoudi E, Abdullah N, Ismail MHS, Mohammad AW, Hussain SA. Synthesis and Characterisation of Graphene Oxide-Silica-Chitosan for Eliminating the Pb(II) from Aqueous Solution. Polymers. 2020; 12(9):1922. https://doi.org/10.3390/polym12091922
Chicago/Turabian StyleAzizkhani, Sepehr, Ebrahim Mahmoudi, Norhafizah Abdullah, Mohd Halim Shah Ismail, Abdul Wahab Mohammad, and Siti Aslina Hussain. 2020. "Synthesis and Characterisation of Graphene Oxide-Silica-Chitosan for Eliminating the Pb(II) from Aqueous Solution" Polymers 12, no. 9: 1922. https://doi.org/10.3390/polym12091922