Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy
Abstract
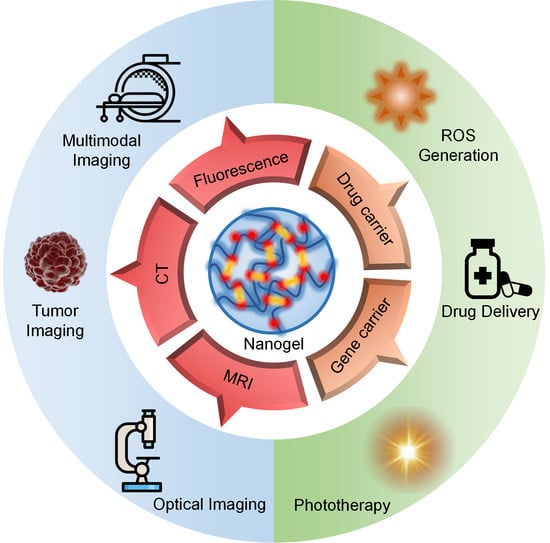
:1. Introduction
2. Preparation of Crosslinked Nanogels
3. Crosslinked Nanogels for Cancer Therapy
3.1. Chemotherapy
3.1.1. Environmentally Responsive Nanogels for Chemotherapy
3.1.2. Drug-Crosslinked Nanogels
3.2. Gene Therapy
3.2.1. Gene-Crosslinked Nanogels
3.2.2. Environmental Responsive Nanogels for Gene Therapy
3.3. Enzyme Dynamic Therapy
4. Crosslinked Nanogels for Cancer Diagnosis and Imaging-Guided Cancer Therapy
4.1. Cancer Imaging
4.2. Imaging-Guided Cancer Therapy
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.C.; Pu, K.Y. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Zhang, W.Z.; Zhu, G.Z.; Xie, J.; Chen, X.Y. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.Z.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Ng, K.K.; Zheng, G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015, 115, 11012–11042. [Google Scholar] [CrossRef]
- Ali, I.; Alsehli, M.; Scotti, L.; Scotti, M.T.; Tsai, S.T.; Yu, R.S.; Hsieh, M.F.; Chen, J.C. Progress in Polymeric Nano-Medicines for Theranostic Cancer Treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef] [Green Version]
- Li, J.C.; Cui, D.; Huang, J.G.; He, S.S.; Yang, Z.B.; Zhang, Y.; Luo, Y.; Pu, K.Y. Organic Semiconducting Pro-nanostimulants for Near-Infrared Photoactivatable Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 12680–12687. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Pu, K.Y. Multimodal Biophotonics of Semiconducting Polymer Nanoparticles. Acc. Chem. Res. 2018, 51, 1840–1849. [Google Scholar] [CrossRef]
- Yin, C.; Zhen, X.; Fan, Q.L.; Huang, W.; Pu, K.Y. Degradable Semiconducting Oligomer Amphiphile for Ratiometric Photoacoustic Imaging of Hypochlorite. ACS Nano 2017, 11, 4174–4182. [Google Scholar] [CrossRef]
- Zhang, W.S.; Deng, W.X.; Zhang, H.; Sun, X.L.; Huang, T.; Wang, W.J.; Sun, P.F.; Fan, Q.L.; Huang, W. Bioorthogonal-targeted 1064 nm excitation theranostic nanoplatform for precise NIR-IIa fluorescence imaging guided efficient NIR-II photothermal therapy. Biomaterials 2020, 243, 119934. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.Z.; Geng, R.Y.; Cai, J.; Li, J.; Xie, C.; Tang, W.H.; Shen, Q.M.; Huang, W.; Fan, Q.L. High performance one-for-all phototheranostics: NIR-II fluorescence imaging guided mitochondria-targeting phototherapy with a single-dose injection and 808 nm laser irradiation. Biomaterials 2020, 231, 119671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ning, L.L.; Huang, J.G.; Zhang, C.; Pu, K.Y. Activatable molecular agents for cancer theranostics. Chem. Sci. 2020, 11, 618–630. [Google Scholar] [CrossRef] [Green Version]
- Zhen, X.; Zhang, J.J.; Huang, J.G.; Xie, C.; Miao, Q.Q.; Pu, K.Y. Macrotheranostic Probe with Disease-Activated Near-Infrared Fluorescence, Photoacoustic, and Photothermal Signals for Imaging-Guided Therapy. Angew. Chem. Int. Ed. 2018, 57, 7804–7808. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Huang, J.G.; Zhen, X.; Li, J.C.; Jiang, Y.Y.; Pu, K.Y. A Semiconducting Polymer Nano-prodrug for Hypoxia-Activated Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 5920–5924. [Google Scholar] [CrossRef]
- He, S.S.; Xie, C.; Jiang, Y.Y.; Pu, K.Y. An Organic Afterglow Protheranostic Nanoassembly. Adv. Mater. 2019, 31, 1902672. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Deng, D.; Xiao, Z.; Dong, Z.; Wang, Z.; Lei, Q.; Gao, S.; Huang, G.; Zhang, E.; et al. Fluorinated Chitosan To Enhance Transmucosal Delivery of Sonosensitizer-Conjugated Catalase for Sonodynamic Bladder Cancer Treatment Post-intravesical Instillation. ACS Nano 2020, 14, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.Y.; Knox, H.J.; Reinhardt, C.J.; Partipilo, G.; Nilges, M.J.; Chan, J. Near-Infrared Photoactivatable Nitric Oxide Donors with Integrated Photoacoustic Monitoring. J. Am. Chem. Soc. 2018, 140, 11686–11697. [Google Scholar] [CrossRef]
- Huang, J.G.; Pu, K.Y. Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2020, 59, 11717–11731. [Google Scholar] [CrossRef]
- Hu, X.M.; Tang, Y.F.; Hu, Y.X.; Lu, F.; Lu, X.M.; Wang, Y.Q.; Li, J.; Li, Y.Y.; Ji, Y.; Wang, W.J.; et al. Gadolinium-Chelated Conjugated Polymer-Based Nanotheranostics for Photoacoustic/Magnetic Resonance/NIR-II Fluorescence Imaging-Guided Cancer Photothermal Therapy. Theranostics 2019, 9, 4168–4181. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, Y.N.; Xu, J.Z.; Cai, J.; Niu, X.R.; Zhang, L.; Chen, R.F.; Shen, Q.M.; Huang, W.; Fan, Q.L. All-in-One Phototheranostics: Single Laser Triggers NIR-II Fluorescence/Photoacoustic Imaging Guided Photothermal/Photodynamic/Chemo Combination Therapy. Adv. Funct. Mater. 2019, 29, 1901480. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.Y. Semiconducting Perylene Diimide Nanostructure: Multifunctional Phototheranostic Nanoplatform. Acc. Chem. Res. 2019, 52, 1245–1254. [Google Scholar] [CrossRef]
- Xie, C.; Upputuri, P.K.; Zhen, X.; Pramanik, M.; Pu, K. Self-quenched semiconducting polymer nanoparticles for amplified in vivo photoacoustic imaging. Biomaterials 2016, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, R.C.; Wang, Q.; Li, X.; Hu, X.M.; Yuan, Y.; Lu, X.M.; Wang, W.J.; Huang, W.; Fan, Q.L. Semiconducting polymer nanotheranostics for NIR-II/Photoacoustic imaging-guided photothermal initiated nitric oxide/photothermal therapy. Biomaterials 2019, 217, 119304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Yin, Z.; Zhang, H.; Gao, Y.; Huo, G.; Wu, A.; Zeng, L. Amplified Photoacoustic Signal and Enhanced Photothermal Conversion of Polydopamine-Coated Gold Nanobipyramids for Phototheranostics and Synergistic Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 14866–14875. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.P.; Zhang, W.Z.; Yang, Z.; Li, L.; Wang, Z.T.; Chiang, Y.L.; Liu, Y.J.; Deng, L.M.; He, L.C.; et al. Wet/Sono-Chemical Synthesis of Enzymatic Two-Dimensional MnO2 Nanosheets for Synergistic Catalysis-Enhanced Phototheranostics. Adv. Mater. 2019, 31, 19000401. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhong, X.Y.; Lei, H.L.; Geng, Y.H.; Zhao, Q.; Gong, F.; Yang, Z.J.; Dong, Z.L.; Liu, Z.; Cheng, L. Hollow Cu2Se Nanozymes for Tumor Photothermal-Catalytic Therapy. Chem. Mater. 2019, 31, 6174–6186. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.W.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Zhu, W.; Chu, J.; Xu, J.; Zhao, H.; Shen, F.; Peng, R.; Liu, Z. Photosensitizer-Modified MnO2 Nanoparticles to Enhance Photodynamic Treatment of Abscesses and Boost Immune Protection for Treated Mice. Small 2020, 16, 2000589. [Google Scholar] [CrossRef]
- Lin, Z.X.; Jiang, B.P.; Liang, J.Z.; Wen, C.C.; Shen, X.C. Phycocyanin functionalized single-walled carbon nanohorns hybrid for near-infrared light-mediated cancer phototheranostics. Carbon 2019, 143, 814–827. [Google Scholar] [CrossRef]
- Hu, D.R.; Chen, L.J.; Qu, Y.; Peng, J.R.; Chu, B.Y.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.Y.; Qian, Z.Y. Oxygen-generating Hybrid Polymeric Nanoparticles with Encapsulated Doxorubicin and Chlorin e6 for Trimodal Imaging-Guided Combined Chemo-Photodynamic Therapy. Theranostics 2018, 8, 1558–1574. [Google Scholar] [CrossRef]
- Fusco, L.; Gazzi, A.; Peng, G.T.; Shin, Y.; Vranic, S.; Bedognetti, D.; Vitale, F.; Yilmazer, A.; Feng, X.; Fadeel, B.; et al. Graphene and other 2D materials: A multidisciplinary analysis to uncover the hidden potential as cancer theranostics. Theranostics 2020, 10, 5435–5488. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, X.; Wen, G.; Yang, B.; Zhang, Y.; Chen, X.; Zhao, P.; Li, S.; Li, R.; Wang, L.; et al. Organic semiconducting polymer amphiphile for near-infrared-II light-triggered phototheranostics. Biomaterials 2020, 232, 119684. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fan, W.P.; Tang, W.; Shen, Z.Y.; Dai, Y.L.; Song, J.B.; Wang, Z.T.; Liu, Y.; Lin, L.S.; Shan, L.L.; et al. Near-Infrared Semiconducting Polymer Brush and pH/GSH-Responsive Polyoxometalate Cluster Hybrid Platform for Enhanced Tumor-Specific Phototheranostics. Angew. Chem. Int. Ed. 2018, 57, 14101–14105. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Zhou, L.Y.; Gu, Q.; Liu, L.B.; Lv, F.T.; Wang, S. Conjugated Polymer Nanoparticles with Appended Photo-Responsive Units for Controlled Drug Delivery, Release, and Imaging. Angew. Chem. Int. Ed. 2018, 57, 13114–13119. [Google Scholar] [CrossRef]
- Zhen, X.; Xie, C.; Pu, K.Y. Temperature-Correlated Afterglow of a Semiconducting Polymer Nanococktail for Imaging-Guided Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, Z.L.; Shen, Y.Q.; Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Kang, N.; Perron, M.E.; Prud’homme, R.E.; Zhang, Y.B.; Gaucher, G.; Leroux, J.C. Stereocomplex block copolymer micelles: Core-shell nanostructures with enhanced stability. Nano Lett. 2005, 5, 315–319. [Google Scholar] [CrossRef]
- O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef]
- Huang, H.Y.; Remsen, E.E.; Kowalewski, T.; Wooley, K.L. Nanocages derived from shell cross-linked micelle templates. J. Am. Chem. Soc. 1999, 121, 3805–3806. [Google Scholar] [CrossRef]
- Thurmond, K.B.; Kowalewski, T.; Wooley, K.L. Water-soluble knedel-like structures: The preparation of shell-cross-linked small particles. J. Am. Chem. Soc. 1996, 118, 7239–7240. [Google Scholar] [CrossRef]
- Tian, S.; Liu, G.; Wang, X.; Zhang, G.; Hu, J. pH-Responsive Tumor-Targetable Theranostic Nanovectors Based on Core Crosslinked (CCL) Micelles with Fluorescence and Magnetic Resonance (MR) Dual Imaging Modalities and Drug Delivery Performance. Polymers 2016, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.P.; Rippe, M.; Companhoni, M.V.P.; Stefanello, T.F.; Louage, B.; Van Herck, S.; Sancey, L.; Coll, J.L.; De Geest, B.G.; Vataru Nakamura, C.; et al. A versatile method for the selective core-crosslinking of hyaluronic acid nanogels via ketone-hydrazide chemistry: From chemical characterization to in vivo biodistribution. Biomater. Sci. 2018, 6, 1754–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, H.Y.; Sanoj Rejinold, N.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.Q.; Pu, K.Y. Organic Semiconducting Agents for Deep-Tissue Molecular Imaging: Second Near-Infrared Fluorescence, Self-Luminescence, and Photoacoustics. Adv. Mater. 2018, 30, 1801778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Xie, C.; Chen, P.; Pu, K.Y. Organic Nanotheranostics for Photoacoustic Imaging-Guided Phototherapy. Curr. Med. Chem. 2019, 26, 1389–1405. [Google Scholar] [CrossRef]
- Cheng, P.; Pu, K. Activatable Phototheranostic Materials for Imaging-Guided Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5286–5299. [Google Scholar] [CrossRef]
- Xie, C.; Yang, C.C.; Zhang, P.; Zhang, J.L.; Wu, W.; Jiang, X.Q. Synthesis of drug-crosslinked polymer nanoparticles. Polym. Chem. 2015, 6, 1703–1713. [Google Scholar] [CrossRef]
- Qian, Q.H.; Shi, L.L.; Gao, X.H.; Ma, Y.; Yang, J.P.; Zhang, Z.H.; Qian, J.W.; Zhu, X.Y. A Paclitaxel-Based Mucoadhesive Nanogel with Multivalent Interactions for Cervical Cancer Therapy. Small 2019, 15, 1903208. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.J.; Wang, J.J.; Wang, X.; Wu, C.; Chen, M.W.; Wu, Q.; Lesniak, M.S.; Mi, Y.L.; Cheng, Y.; et al. A Neutrophil-Inspired Supramolecular Nanogel for Magnetocaloric-Enzymatic Tandem Therapy. Angew. Chem. Int. Ed. 2020, 59, 3732–3738. [Google Scholar] [CrossRef]
- Ding, F.; Mou, Q.B.; Ma, Y.; Pan, G.F.; Guo, Y.Y.; Tong, G.S.; Choi, C.H.J.; Zhu, X.Y.; Zhang, C. A Crosslinked Nucleic Acid Nanogel for Effective siRNA Delivery and Antitumor Therapy. Angew. Chem. Int. Ed. 2018, 57, 3064–3068. [Google Scholar] [CrossRef]
- Qian, H.Q.; Wang, X.; Yuan, K.J.; Xie, C.; Wu, W.; Jiang, X.Q.; Hu, L.J. Delivery of doxorubicin in vitro and in vivo using bio-reductive cellulose nanogels. Biomater. Sci. 2014, 2, 220–232. [Google Scholar] [CrossRef]
- Wu, Q.; He, Z.G.; Wang, X.; Zhang, Q.; Wei, Q.C.; Ma, S.Q.; Ma, C.; Li, J.; Wang, Q.G. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Gao, L.N.; Zhu, X.N.; Zhang, Y.; Zhang, C.N.; Xu, D.; Cui, Y.L. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics 2019, 9, 6239–6255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Andren, O.C.J.; Nordstrom, R.; Fan, Y.M.; Malmsten, M.; Mongkhontreerat, S.; Malkoch, M. Off-Stoichiometric Thiol-Ene Chemistry to Dendritic Nanogel Therapeutics. Adv. Funct. Mater. 2019, 29, 1806693. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Yi, Z.; Cui, X.X.; Chen, X.Y.; Su, W.; Ren, X.X.; Li, X.D. Tumor-targeted and nitric oxide-generated nanogels of keratin and hyaluronan for enhanced cancer therapy. Nanoscale 2018, 10, 12109–12122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Li, Y.J.; Wan, J.X.; Long, P.H.; Guo, J.; Chen, G.S.; Wang, C.C. Preparation of Pt(IV)-crosslinked polymer nanoparticles with an anti-detoxifying effect for enhanced anticancer therapy. Polym. Chem. 2017, 8, 2410–2422. [Google Scholar] [CrossRef]
- Guo, D.B.; Xu, S.T.; Yasen, W.; Zhang, C.; Shen, J.; Huang, Y.; Chen, D.; Zhu, X.Y. Tirapazamine-embedded polyplatinum(iv) complex: A prodrug combo for hypoxia-activated synergistic chemotherapy. Biomater. Sci. 2020, 8, 694–701. [Google Scholar] [CrossRef]
- Ding, F.; Gao, X.; Huang, X.; Ge, H.; Xie, M.; Qian, J.; Song, J.; Li, Y.; Zhu, X.; Zhang, C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials 2020, 245, 119976. [Google Scholar] [CrossRef]
- Li, H.P.; Yang, X.; Gao, F.; Qian, C.G.; Li, C.Z.; Oupicky, D.; Sun, M.J. Bioreduction-ruptured nanogel for switch on/off release of Bcl2 siRNA in breast tumor therapy. J. Control. Release 2018, 292, 78–90. [Google Scholar] [CrossRef]
- Si, X.H.; Ma, S.; Xu, Y.; Zhang, D.; Shen, N.; Yu, H.Y.; Zhang, Y.; Song, W.T.; Tang, Z.H.; Chen, X. Hypoxia-sensitive supramolecular nanogels for the cytosolic delivery of ribonuclease A as a breast cancer therapeutic. J. Control. Release 2020, 320, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Aktan, B.; Chambre, L.; Sanyal, R.; Sanyal, A. “Clickable” Nanogels via Thermally Driven Self-Assembly of Polymers: Facile Access to Targeted Imaging Platforms using Thiol—Maleimide Conjugation. Biomacromolecules 2017, 18, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Jia, H.R.; Chen, Z.; Wu, F.G. Photosensitizer (PS)/polyhedral oligomeric silsesquioxane (POSS)-crosslinked nanohybrids for enhanced imaging-guided photodynamic cancer therapy. Nanoscale 2017, 9, 12874–12884. [Google Scholar] [CrossRef]
- Peng, S.J.; Wang, H.; Zhao, W.; Xin, Y.J.; Liu, Y.; Yu, X.R.; Zhan, M.X.; Shen, S.; Lu, L.G. Zwitterionic Polysulfamide Drug Nanogels with Microwave Augmented Tumor Accumulation and On-Demand Drug Release for Enhanced Cancer Therapy. Adv. Funct. Mater. 2020, 30, 20001832. [Google Scholar] [CrossRef]
- Zhai, Y.H.; Ran, W.; Su, J.H.; Lang, T.Q.; Meng, J.; Wang, G.R.; Zhang, P.C.; Li, Y.P. Traceable Bioinspired Nanoparticle for the Treatment of Metastatic Breast Cancer via NIR-Trigged Intracellular Delivery of Methylene Blue and Cisplatin. Adv. Mater. 2018, 30, 1802378. [Google Scholar] [CrossRef]
- Xue, Y.A.; Xia, X.Y.; Yu, B.; Tao, L.J.; Wang, Q.; Huang, S.W.; Yu, F.Q. Selenylsulfide Bond-Launched Reduction-Responsive Superparamagnetic Nanogel Combined of Acid-Responsiveness for Achievement of Efficient Therapy with Low Side Effect. ACS Appl. Mater. Interfaces 2017, 9, 30253–30257. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, W.; Zhang, J.; Zhou, Y.; Shen, M.; Peng, C.; Shi, X. Facile Formation of Gold-Nanoparticle-Loaded γ-Polyglutamic Acid Nanogels for Tumor Computed Tomography Imaging. Bioconju. Chem. 2017, 28, 2692–2697. [Google Scholar] [CrossRef]
- Sun, W.J.; Zhang, J.L.; Zhang, C.C.; Wang, P.; Peng, C.; Shen, M.W.; Shi, X.Y. Construction of Hybrid Alginate Nanogels Loaded with Manganese Oxide Nanoparticles for Enhanced Tumor Magnetic Resonance Imaging. ACS Macro Lett. 2018, 7, 137–142. [Google Scholar] [CrossRef]
- Sun, W.J.; Zhang, J.L.; Zhang, C.C.; Zhou, Y.W.; Zhu, J.Z.; Peng, C.; Shen, M.W.; Shi, X.Y. A unique nanogel-based platform for enhanced dual mode tumor MR/CT imaging. J. Mater. Chem. B 2018, 6, 4835–4842. [Google Scholar] [CrossRef]
- Li, Q.; Plao, X.K.; Wang, F.C.; Li, X.J.; Yang, J.; Liu, Y.; Shi, L.Q.; Liu, D.B. Encapsulating a Single Nanoprobe in a Multifunctional Nanogel for High-Fidelity Imaging of Caspase Activity in Vivo. Anal. Chem. 2019, 91, 13633–13638. [Google Scholar] [CrossRef]
- Xiang, H.; Xue, F.; Yi, T.; Tham, H.P.; Liu, J.G.; Zhao, Y. Cu2−xS Nanocrystals Cross-Linked with Chlorin e6-Functionalized Polyethylenimine for Synergistic Photodynamic and Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 16344–16351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Sun, W.J.; Wang, Y.; Xu, F.; Qu, J.; Xia, J.D.; Shen, M.W.; Shi, X.Y. Gd-/CuS-Loaded Functional Nanogels for MR/PA Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 9107–9117. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, D.; Wang, Y.; Ouyang, Z.J.; Peng, Y.C.; Tomas, H.; Xia, J.D.; Rodrigues, J.; Shen, M.W.; Shi, X.Y. Polyethylenimine Nanogels Incorporated with Ultrasmall Iron Oxide Nanoparticles and Doxorubicin for MR Imaging-Guided Chemotherapy of Tumors. Bioconju. Chem. 2020, 31, 907–915. [Google Scholar] [CrossRef]
- Jing, X.; Zhi, Z.; Jin, L.; Wang, F.; Wu, Y.; Wang, D.; Yan, K.; Shao, Y.; Meng, L. pH/redox dual-stimuli-responsive cross-linked polyphosphazene nanoparticles for multimodal imaging-guided chemo-photodynamic therapy. Nanoscale 2019, 11, 9457–9467. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for Drug Delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, S.S.; Goncalves, C.; David, L.; Gama, M. A Novel Crosslinked Hyaluronic Acid Nanogel for Drug Delivery. Macromol. Biosci. 2014, 14, 1556–1568. [Google Scholar] [CrossRef] [Green Version]
- Chacko, R.T.; Ventura, J.; Zhuang, J.M.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sun, S.; Zhang, Z.; Shi, D. Nanomaterials for Cancer Precision Medicine. Adv. Mater. 2018, 30, 1705660. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [Green Version]
- Saito, G.; Swanson, J.A.; Lee, K.D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Tang, M.L.; Zhou, M.L.; Huang, Y.A.; Zhong, J.J.; Zhou, Z.; Luo, K. Dual-sensitive and biodegradable core-crosslinked HPMA copolymer-doxorubicin conjugate-based nanoparticles for cancer therapy. Polym. Chem. 2017, 8, 2370–2380. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Liu, X.R.; Zhu, D.C.; Wang, Y.; Zhang, Z.; Zhou, X.F.; Qiu, N.S.; Chen, X.S.; Shen, Y.Q. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Adv. Drug Deliv. Rev. 2017, 115, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.A. State-of-the-art gene-based therapies: The road ahead. Nat. Rev. Genet. 2011, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, Y.; Bu, W.B.; Cheng, C.; Zuo, C.J.; Xiao, Q.F.; Sun, Y.; Ni, D.L.; Zhang, C.; Liu, J.A.; et al. Hypoxia Induced by Upconversion-Based Photodynamic Therapy: Towards Highly Effective Synergistic Bioreductive Therapy in Tumors. Angew. Chem. Int. Ed. 2015, 54, 8105–8109. [Google Scholar] [CrossRef]
- Yang, S.C.; Tang, Z.H.; Hu, C.Y.; Zhang, D.W.; Shen, N.; Yu, H.Y.; Chen, X.S. Selectively Potentiating Hypoxia Levels by Combretastatin A4 Nanomedicine: Toward Highly Enhanced Hypoxia-Activated Prodrug Tirapazamine Therapy for Metastatic Tumors. Adv. Mater. 2019, 31, 1805955. [Google Scholar] [CrossRef]
- Zabernigg, A.; Gamper, E.M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010, 15, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.M.; Liu, Y.Y.; He, M.Y.; Bu, W.B. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Ding, B.B.; Shao, S.; Jiang, F.; Dang, P.P.; Sun, C.Q.; Huang, S.S.; Ma, P.A.; Jin, D.Y.; Al Kheraif, A.A.; Lin, J. MnO2-Disguised Upconversion Hybrid Nanocomposite: An Ideal Architecture for Tumor Microenvironment-Triggered UCL/MR Bioimaging and Enhanced Chemodynamic Therapy. Chem. Mater. 2019, 31, 2651–2660. [Google Scholar] [CrossRef]
- Fang, C.; Deng, Z.; Cao, G.D.; Chu, Q.; Wu, Y.L.; Li, X.; Peng, X.S.; Han, G.R. Co-Ferrocene MOF/Glucose Oxidase as Cascade Nanozyme for Effective Tumor Therapy. Adv. Funct. Mater. 2020, 30, 1910085. [Google Scholar] [CrossRef]
- Kumar, R.; Han, J.; Lim, H.J.; Ren, W.X.; Lim, J.Y.; Kim, J.H.; Kim, J.S. Mitochondrial Induced and Self-Monitored Intrinsic Apoptosis by Antitumor Theranostic Prodrug: In Vivo Imaging and Precise Cancer Treatment. J. Am. Chem. Soc. 2014, 136, 17836–17843. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, Y.; Imai, M.; Ikegawa, C.; Arai, T. Dedicated breast PET versus whole-body PET/CT: A comparative study. J. Nucl. Med. 2018, 59, 1582. [Google Scholar]
- Zhou, W.; Chen, Y.; Zhang, Y.T.; Xin, X.Y.; Li, R.T.; Xie, C.; Fan, Q.L. Iodine-Rich Semiconducting Polymer Nanoparticles for CT/Fluorescence Dual-Modal Imaging-Guided Enhanced Photodynamic Therapy. Small 2020, 16, 1905641. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.L.; Ehlerding, E.B.; Cai, W.B. Multimodality Imaging Agents with PET as the Fundamental Pillar. Angew. Chem. Int. Ed. 2019, 58, 2570–2579. [Google Scholar] [CrossRef]
- Wang, C.; Fan, W.P.; Zhang, Z.J.; Wen, Y.; Xiong, L.; Chen, X.Y. Advanced Nanotechnology Leading the Way to Multimodal Imaging-Guided Precision Surgical Therapy. Adv. Mater. 2019, 31, 1904329. [Google Scholar] [CrossRef]
- Miao, Q.Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.W.; Liu, X.G.; Jokerst, J.V.; Pu, K.Y. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102–1110. [Google Scholar] [CrossRef]
- Xie, C.; Lyu, Y.; Zhen, X.; Miao, Q.; Pu, K. Activatable Semiconducting Oligomer Amphiphile for Near-Infrared Luminescence Imaging of Biothiols. ACS Appl. Bio. Mater. 2018, 1, 1147–1153. [Google Scholar] [CrossRef]
- Park, S.M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef]
- Li, J.C.; Zhen, X.; Lyu, Y.; Jiang, Y.Y.; Huang, J.G.; Pu, K.Y. Cell Membrane Coated Semiconducting Polymer Nanoparticles for Enhanced Multimodal Cancer Phototheranostics. ACS Nano 2018, 12, 8520–8530. [Google Scholar] [CrossRef]
- Zhen, X.; Cheng, P.H.; Pu, K.Y. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, 1804105. [Google Scholar] [CrossRef] [Green Version]
- An, H.W.; Li, L.L.; Wang, Y.; Wang, Z.Q.; Hou, D.Y.; Lin, Y.X.; Qiao, S.L.; Wang, M.D.; Yang, C.; Cong, Y.; et al. A tumour-selective cascade activatable self-detained system for drug delivery and cancer imaging. Nat. Commun. 2019, 10, 4861. [Google Scholar] [CrossRef]
- Lyu, Y.; Fang, Y.; Miao, Q.Q.; Zhen, X.; Ding, D.; Pu, K.Y. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wu, Y.X.; Chen, J.T.; Wan, J.L.; Xiao, C.; Guan, J.K.; Song, X.L.; Li, S.Y.; Zhang, M.M.; Cui, H.C.; et al. A Simple Glutathione-Responsive Turn-On Theranostic Nanoparticle for Dual-Modal Imaging and Chemo-Photothermal Combination Therapy. Nano Lett. 2019, 19, 5806–5817. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Hui, Y.; Middelberg, A.P.J.; Zhao, C.X. Interfacial engineering for silica nanocapsules. Adv. Colloid Interfac. 2016, 236, 83–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Miro, M.; Chen, S.X.; Gonzaga, Z.J.; Evert, B.; Wibowo, D.; Rehm, B.H.A. Polyester as Antigen Carrier toward Particulate Vaccines. Biomacromolecules 2019, 20, 3213–3232. [Google Scholar] [CrossRef]











| Type | Inorganic Core | Materials | Crosslinker | Crosslinking Interaction | References |
|---|---|---|---|---|---|
| Organic | Alginate | Ca ion | Electrostatic interaction | [54] | |
| Dendritic blocks | TMP-SH | Covalent bond | [55] | ||
| Hyaluronic acid | Keratin | Hydrogen bond | [56] | ||
| PAA-βCD | PAA-TAX | Supramolecular interaction | [49] | ||
| - | Pt(IV) prodrug | Covalent bond | [57] | ||
| - | PPM | Covalent bond | [58] | ||
| DNA-g-PCL | siRNA | Electrostatic interaction | [51] | ||
| DNA-g-PCL | siRNA | Electrostatic interaction | [59] | ||
| Dextrin-SH | PEI-SH | Covalent bond | [60] | ||
| PLG-g-mPEG/βCD | PLG-g-mPEG/Azo | Supramolecular interaction | [61] | ||
| poly(PEGMEMA-co-MaMA) | 2,2′-(ethylenedioxy)diethanethiol | Covalent bond | [62] | ||
| POSS | Ce6 | Covalent bond | [63] | ||
| MEDAPA | EGDMA/BAC | Covalent bond | [64] | ||
| Gelatin | glutaraldehyde | Covalent bond | [65] | ||
| Inorganic | MNP-NH2 | Alginate | - | Covalent bond | [66] |
| MNPs | Tyrosine | - | π-π interaction | [53] | |
| MNPs | Tyrosine | - | π-π interaction | [50] | |
| AuNPs | γ-PGA | PEI | Covalent bond | [67] | |
| Mn3O4 NPs | Alginate | PEI | Covalent bond | [68] | |
| AuNPs | Alginate | PEI | Covalent bond | [69] | |
| AuNPs | Cy5/vinyl-labeled peptide | Glycerol dimethacrylate | Covalent bond | [70] | |
| Cu2−xS NPs | PEI | Ce6 | Covalent bond | [71] | |
| CuS NPs | PEI | BIS | Covalent bond | [72] | |
| Fe3O4 NPs | PEI | BIS | Covalent bond | [73] | |
| Fe3O4 NPs | HCCP | CUR/HPS | Covalent bond | [74] |
| Type | Loaded Drug | Loading Capacity | Responsiveness | Animal Study | References |
|---|---|---|---|---|---|
| Chemotherapy | DOX/GL | 1.2% (DOX) | pH | Yes | [54] |
| DOX | 5.7% | - | No | [55] | |
| DOX | 54.1% | pH/GSH/trypsin | Yes | [56] | |
| DOX | 18.2% | pH/GSH | Yes | [66] | |
| TAX | 20–30% | pH/esterase | Yes | [49] | |
| Pt(IV) | 60.8% | GSH/ascorbic acid | Yes | [57] | |
| Pt(IV)/TPZ | 8.06% (Pt)/9.12% (TPZ) | GSH | Yes | [58] | |
| Gene therapy | siRNA | - | RNase H | Yes | [51] |
| siRNA | - | pH/RNase H | Yes | [59] | |
| siBcl2 | - | DTT | Yes | [60] | |
| RNase | 23.5% | NTR | Yes | [61] | |
| Enzyme dynamic therapy | - | - | ·O2−/H2O2 | Yes | [53] |
| - | - | H2O2 | Yes | [50] |
| Type | Imaging Modality | Therapeutic Method | Responsiveness | Animal Study | References |
|---|---|---|---|---|---|
| Imaging | CT | - | - | Yes | [67] |
| T1-MRI | - | - | Yes | [68] | |
| T1-MRI/CT | - | - | Yes | [69] | |
| FL | - | - | No | [62] | |
| FL | - | pH/caspases | Yes | [70] | |
| Imaging-guided therapy | FL | PDT/PTT | - | Yes | [71] |
| FL | PDT | - | Yes | [63] | |
| FL | Chemotherapy | Temperature | Yes | [64] | |
| T1-MRI/PA | PTT | - | Yes | [72] | |
| T1-MRI | Chemotherapy | pH | Yes | [73] | |
| PA/FL/PT | PDT/chemotherapy | Laser | Yes | [65] | |
| Fluorescence/T2-MRI | PDT/chemotherapy | pH/GSH | Yes | [74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Yang, G.; Ni, X.; Diao, S.; Xie, C.; Fan, Q. Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy. Polymers 2020, 12, 1902. https://doi.org/10.3390/polym12091902
Zhou W, Yang G, Ni X, Diao S, Xie C, Fan Q. Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy. Polymers. 2020; 12(9):1902. https://doi.org/10.3390/polym12091902
Chicago/Turabian StyleZhou, Wen, Guangzhao Yang, Xiaoyue Ni, Shanchao Diao, Chen Xie, and Quli Fan. 2020. "Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy" Polymers 12, no. 9: 1902. https://doi.org/10.3390/polym12091902