Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse
Abstract
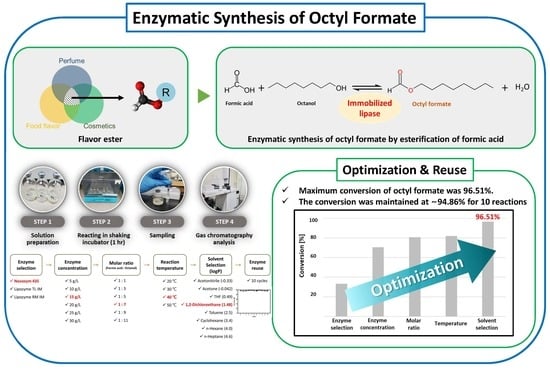
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methodology
2.3. Sample Preparation
2.4. Enzyme Reuse
2.5. Sample Analysis
3. Results and Discussion
3.1. Effects of Experimental Variables on Enzymatic Synthesis of Octyl Formate
3.1.1. Enzyme Selection
3.1.2. Enzyme Concentration
3.1.3. Molar Ratio of Reactants
3.1.4. Effect of Temperature
3.1.5. Variations in Solvent
3.2. Reusability of Selected Lipase for Synthesis of Octyl Formate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berger, R.G.; De Bont, J.A.M.; Eggink, G.; Da Fonseca, M.M.; Gehrke, M.; Gros, J.-B.; Van Keulen, F.; Krings, U.; Larroche, C.; Leak, D.J.; et al. Biotransformations in the flavour industry. In Current Topics in Flavours and Fragrances: Towards a New Millennium of Discovery; Swift, K.A.D., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 139–170. ISBN 9789401140225. [Google Scholar]
- Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=2565 (accessed on 10 October 2019).
- Food Flavors Market to Reach USD 19.72 Billion by 2026|Reports and Data. Available online: https://www.reportsanddata.com/report-detail/food-flavors-market#utm_source=globenewswire&utm_medium=referral&utm_campaign=shuv10oct2019&utm_content=DP (accessed on 10 October 2019).
- Shin, M.; Seo, J.; Baek, Y.; Lee, T.; Jang, M.; Park, C. Novel and efficient synthesis of phenethyl formate via enzymatic esterification of formic acid. Biomolecules 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatfield, I.L. Enzymatic and microbial generation of flavors. Perfum. Flavor. 1995, 20, 5–14. [Google Scholar]
- Garlapati, V.K.; Banerjee, R. Solvent-free synthesis of flavour esters through immobilized lipase mediated transesterification. Enzyme Res. 2013, 2013, 367410. [Google Scholar] [CrossRef] [Green Version]
- Cha, H.-J.; Park, J.-B.; Park, S. Esterification of secondary alcohols and multi-hydroxyl compounds by Candida antarctica lipase B and subtilisin. Biotechnol. Bioprocess Eng. 2019, 24, 41–47. [Google Scholar] [CrossRef]
- Won, Y.; Pagar, A.D.; Patil, M.D.; Dawson, P.E.; Yun, H. Recent advances in enzyme engineering through incorporation of unnatural amino acids. Biotechnol. Bioprocess Eng. 2019, 24, 592–604. [Google Scholar] [CrossRef]
- Janssen, L.M.G.; van Oosten, R.; Paul, C.E.; Arends, I.W.C.E.; Hollmann, F. Lipase-catalyzed transesterification of ethyl formate to octyl formate. J. Mol. Catal. B Enzyme 2014, 105, 7–10. [Google Scholar] [CrossRef]
- Stergiou, P.-Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Ghamgui, H.; Karra-Chaâbouni, M.; Bezzine, S.; Miled, N.; Gargouri, Y. Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzyme Microb. Technol. 2006, 38, 788–794. [Google Scholar] [CrossRef]
- Torres, S.; Baigorí, M.D.; Swathy, S.L.; Pandey, A.; Castro, G.R. Enzymatic synthesis of banana flavour (isoamyl acetate) by Bacillus licheniformis S-86 esterase. Food Res. Int. 2009, 42, 454–460. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Karanth, N.G. Lipase-catalyzed synthesis of isoamyl butyrate. Biochim. Biophys. Acta 2001, 1547, 262–267. [Google Scholar] [CrossRef]
- Claon, P.A.; Akoh, C.C. Enzymatic synthesis of geranyl acetate in n-hexane with Candida antarctica lipases. J. Am. Oil Chem. Soc. 1994, 71, 575–578. [Google Scholar] [CrossRef]
- Claon, P.A.; Akoh, C.C. Effect of reaction parameters on SP435 lipase-catalyzed synthesis of citronellyl acetate in organic solvent. Enzyme Microb. Technol. 1994, 16, 835–838. [Google Scholar] [CrossRef]
- Yadav, G.D.; Trivedi, A.H. Kinetic modeling of immobilized-lipase catalyzed transesterification of n-octanol with vinyl acetate in non-aqueous media. Enzyme Microb. Technol. 2003, 32, 783–789. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Hong, Y.-J.; Yoon, S.H. Enantiomeric synthesis of (S)-2-methylbutanoic acid methyl ester, apple flavor, using lipases in organic solvent. J. Agric. Food Chem. 2000, 48, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Saxena, D.K.; Naik, S.N. Synthesis of food flavors by enzymatic esterification process. Int. J. Sci. Res. 2014, 3, 2113–2116. [Google Scholar]
- Güvenç, A.; Kapucu, N.; Mehmetoğlu, Ü. The production of isoamyl acetate using immobilized lipases in a solvent-free system. Process Biochem. 2002, 38, 379–386. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [Green Version]
- Verdasco-Martín, C.M.; Villalba, M.; dos Santos, J.C.S.; Tobajas, M.; Fernandez-Lafuente, R.; Otero, C. Effect of chemical modification of Novozym 435 on its performance in the alcoholysis of camelina oil. Biochem. Eng. J. 2016, 111, 75–86. [Google Scholar] [CrossRef]
- SÁ, A.G.A.; Meneses, A.C.; Araujo, P.H.H.; Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Immobilized Lipase for Biocatalysis for Smart Chemical Synthesis, Novozymes. Available online: http://www.novozymes.com/en (accessed on 13 June 2016).
- Gu, J.; Xin, Z.; Meng, X.; Sun, S.; Qiao, Q.; Deng, H. Studies on biodiesel production from DDGS-extracted corn oil at the catalysis of Novozym 435/super absorbent polymer. Fuel 2015, 146, 33–40. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, D.-H.; Chen, N.; Zhi, G.-Y. Synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. Bioresour. Technol. 2015, 198, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.D.; Devendran, S. Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process Biochem. 2012, 47, 496–502. [Google Scholar] [CrossRef]
- Sun, W.-J.; Zhao, H.-X.; Cui, F.-J.; Li, Y.-H.; Yu, S.-L.; Zhou, Q.; Qian, J.-Y.; Dong, Y. D-isoascorbyl palmitate: Lipase-catalyzed synthesis, structural characterization and process optimization using response surface methodology. Chem. Cent. J. 2013, 7, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezbradica, D.; Mijin, D.; Siler-Marinkovic, S.; Knezevic, Z. The Candida rugosa lipase catalyzed synthesis of amyl isobutyrate in organic solvent and solvent-free system: A kinetic study. J. Mol. Catal. B Enzyme 2006, 38, 11–16. [Google Scholar] [CrossRef]
- Deng, L.; Xu, X.; Haraldsson, G.G.; Tan, T.; Wang, F. Enzymatic production of alkyl esters through alcoholysis: A critical evaluation of lipases and alcohols. J. Am. Oil Chem. Soc. 2005, 82, 341–347. [Google Scholar] [CrossRef]
- Liu, S.; Nie, K.; Zhang, X.; Wang, M.; Deng, L.; Ye, X.; Wang, F.; Tan, T. Kinetic study on lipase-catalyzed biodiesel production from waste cooking oil. J. Mol. Catal. B Enzyme 2014, 99, 43–50. [Google Scholar] [CrossRef]
- Bovara, R.; Carrea, G.; Ottolina, G.; Riva, S. Water activity does not influence the enantioselectivity of Lipase PS and lipoprotein lipase in organic solvents. Biotechnol. Lett. 1993, 15, 169–174. [Google Scholar] [CrossRef]
- Lima, V.M.G.; Krieger, N.; Mitchell, D.A.; Fontana, J.D. Activity and stability of a crude lipase from Penicillium aurantiogriseum in aqueous media and organic solvents. Biochem. Eng. J. 2004, 18, 65–71. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Xie, Y.; Yi, Y.; Li, C.; Yan, Y.; Liu, Y. Esterification activity and conformation studies of Burkholderia cepacia lipase in conventional organic solvents, ionic liquids and their co-solvent mixture media. Bioresour. Technol. 2010, 101, 9822–9824. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Zhou, H.; Liu, Y.; Secundo, F.; Liu, Y. Enzyme stability and activity in non-aqueous reaction systems: A mini review. Catalyst 2016, 6, 32. [Google Scholar] [CrossRef]
- Graebin, N.G.; Martins, A.B.; Lorenzoni, A.S.G.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads improves butyl acetate synthesis. Biotechnol. Progress 2012, 28, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.B.; Schein, M.F.; Friedrich, J.L.R.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Ultrasound-assisted butyl acetate synthesis catalyzed by Novozym 435: Enhanced activity and operational stability. Ultrason. Sonochem. 2013, 20, 1155–1160. [Google Scholar] [CrossRef] [PubMed]







| Immobilized Enzyme | Support | Activity | Optimal Temp. (°C) | Specific Substrate | Regioselectivity |
|---|---|---|---|---|---|
| Novozym 435 | Lewatit vp oc 1600 | 10,000 PLU/g | 30–60 | Esters and alcohols | Nonspecific |
| Lipozyme RM IM | Duolite ES 562 | 275 IUN/g | 30–50 | Esters | 1,3-specific |
| Lipozyme TL IM | Gel silicate | 250 IUN/g | 50–75 | Esters | 1,3-specific |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, Y.; Lee, J.; Son, J.; Lee, T.; Sobhan, A.; Lee, J.; Koo, S.-M.; Shin, W.H.; Oh, J.-M.; Park, C. Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse. Polymers 2020, 12, 1802. https://doi.org/10.3390/polym12081802
Baek Y, Lee J, Son J, Lee T, Sobhan A, Lee J, Koo S-M, Shin WH, Oh J-M, Park C. Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse. Polymers. 2020; 12(8):1802. https://doi.org/10.3390/polym12081802
Chicago/Turabian StyleBaek, Yesol, Jonghwa Lee, Jemin Son, Taek Lee, Abdus Sobhan, Jinyoung Lee, Sang-Mo Koo, Weon Ho Shin, Jong-Min Oh, and Chulhwan Park. 2020. "Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse" Polymers 12, no. 8: 1802. https://doi.org/10.3390/polym12081802