Physical Properties of Carboxymethyl Cellulose from Palm Bunch and Bagasse Agricultural Wastes: Effect of Delignification with Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Cellulose Extraction and Bleaching
2.4. Synthesis of CMC
2.5. Characterization of Cellulose and CMC Powder
2.6. Preparation of CMC Films
2.7. Properties of CMC Films
2.8. Statistical Analysis
3. Results
3.1. Characterization of Cellulose
3.2. Characterization of CMC
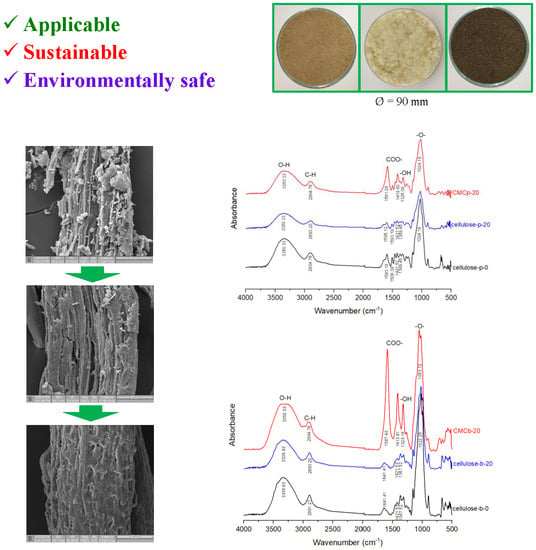
3.3. Morphology of Cellulose and CMC
3.4. FTIR Spectroscopy of Cellulose and CMC
3.5. Characterization of CMC Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Yang, J.; Zeng, Q.; Huang, Y. Effect of carboxymethyl cellulose addition on the properties of Si3N4 ceramic foams. Ceram. Int. 2013, 39, 2775–2779. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Alcalà, M.; Merayo, N.; Balea, A.; Blanco, Á.; Mutjé, P.; Delgado-Aguilar, M. Combined effect of sodium carboxymethyl cellulose, cellulose nanofibers and drainage aids in recycled paper production process. Carbohydr. Polym. 2018, 183, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Fijan, R.; Basile, M.; Šostar-Turk, S.; Žagar, E.; Žigon, M.; Lapasin, R. A study of rheological and molecular weight properties of recycled polysaccharides used as thickeners in textile printing. Carbohydr. Polym. 2009, 76, 8–16. [Google Scholar] [CrossRef]
- Singh, V.; Joshi, S.; Malviya, T. Carboxymethyl cellulose-rosin gum hybrid nanoparticles: An efficient drug carrier. Int. J. Biol. Macromol. 2018, 112, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Schuh, V.; Allard, K.; Herrmann, K.; Gibis, M.; Kohlus, R.; Weiss, J. Impact of carboxymethyl cellulose (CMC) and microcrystalline cellulose (MCC) on functional characteristics of emulsified sausages. Meat Sci. 2013, 93, 240–247. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mauricio, Y.; Matsuhiro, B.; Maldonado, S.; González, R.; Luengo, J.; Uyarte, O.; Serafine, D.; Moya, S.; Romero, J.; Torres, R.; et al. Carboxymethylcellulose from bleached organosolv fibers of Eucalyptus nitens: Synthesis and physicochemical characterization. Cellulose 2018, 25, 2901–2914. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT-Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Adinugraha, M.P.; Marseno, D.W.; Hayadi. Synthesis and characterization of sodium carboxymethylcellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Križman, P.; Kovač, F.; Tavčer, P.F. Bleaching of cotton fabric with peracetic acid in the presence of different activators. Color. Technol. 2005, 121, 304–309. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Eitssayeam, S.; Pengpat, K. Study of carboxmethyl cellulose from papaya peels binder in ceramics. Adv. Mat. Res. 2010, 93–94, 17–21. [Google Scholar]
- Rayung, M.; Ibrahim, N.A.; Zainuddin, N.; Saad, W.Z.; Razak, N.I.A.; Chieng, B.W. The effect of fiber bleaching treatment on the properties of poly(lactic acid)/oil palm empty fruit bunch fiber composites. Int. J. Mol. Sci. 2014, 15, 14728–14742. [Google Scholar] [CrossRef] [Green Version]
- Topalovic, T.; Nierstrasz, V.A.; Bautista, L.; Jocic, D.; Navarro, A.; Warmoeskerken, M.M.C.G. Analysis of the effects of catalytic bleaching on cotton. Cellulose 2007, 14, 385–400. [Google Scholar] [CrossRef] [Green Version]
- Henniges, U.; Potthast, A. Bleaching Revisited: Impact of Oxidative and Reductive Bleaching Treatments on Cellulose and Paper. Restaurator 2009, 30, 294–320. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Alanazi, H.H.; Alghamdi, A.A. Extraction and bleaching of olive tree branch cellulose. BioResources 2015, 10, 7136–7150. [Google Scholar] [CrossRef] [Green Version]
- Jannah, M.; Ahmad, A.; Hayatun, A.; Taba, P.; Chadijah, S. Effect of filler and plastisizer on the mechanical properties of bioplastic cellulose from rice husk. J. Phys. Conf. Ser. 2019, 1341. [Google Scholar] [CrossRef] [Green Version]
- Togrul, H.; Arslan, N. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behavior of carboxymethyl cellulose. Carbohydr. Polym. 2003, 54, 73–82. [Google Scholar] [CrossRef]
- Lin, X.; Qu, T.; Qi, S. Kinetics of the carboxymethyl cellulose in the isopropyl alcohol system. Acta Polym. 1990, 41, 220. [Google Scholar]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethylcellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Kumthai, S.; Mulkarat, N.; Pintajam, N.; Suriyatem, R. Value added of mulberry paper waste by carboxymethylation for preparation a packaging film. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012081. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.I.H.; Yeasmin, M.S.; Rahman, M.S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Utilization of Carboxymethyl Cellulose from Durian Rind Agricultural Waste to Improve Physical Properties and Stability of Rice Starch-Based Film. J. Polym. Environ. 2019, 27, 286–298. [Google Scholar] [CrossRef]
- TAPPI. Alpha-, Beta- and Gamma-Cellulose in Pulp. In Test Method T 203 cm-09, 2009. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T203.aspx (accessed on 11 June 2020).
- TAPPI. Acid-Insoluble Lignin in Wood and Pulp. In Test Method T 222 om-15, 2015. Available online: https://www.techstreet.com/standards/tappi-t-222-om-15?product_id=2000142 (accessed on 11 June 2020).
- TAPPI. Kappa Number of Pulp. In Test Method T 236 om-99, 1999. Available online: https://research.cnr.ncsu.edu/wpsanalytical/documents/T236.PDF (accessed on 11 June 2020).
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of mechanical properties and thermal stability of biodegradable rice starch–based films blended with carboxymethyl chitosan. Ind. Crop. Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- ASTM-D882-12. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2012; Available online: https://www.scribd.com/document/383783567/ASTM-D882-12 (accessed on 11 June 2020).
- ASTM-E96/E9M-16. Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2016; Available online: https://www.astm.org/Standards/E96 (accessed on 11 June 2020).
- Coral Medina, J.D.; Woiciechowski, A.; Zandona Filho, A.; Noseda, M.D.; Kaur, B.S.; Soccol, C.R. Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment—A biorefinery approach. Bioresour. Technol. 2015, 194, 172–178. [Google Scholar] [CrossRef]
- Phinichka, N.; Kaenthong, S. Regenerated cellulose from high alpha cellulose pulp of steam-exploded sugarcane bagasse. J. Mater. Res. Technol. 2018, 7, 55–65. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.K.; Onsrithong, N.; Suwankrua, R.; Jonglertjunya, W. Improving enzymatic saccharification of sugarcane bagasse by biological/physico-chemical pretreatment using Trametes versicolor and Bacillus sp. Bioresour. Bioprocess. 2012, 7, 3935–3947. [Google Scholar]
- Masarin, F.; Gurpilhares, D.B.; Baffa, D.C.; Barbosa, M.H.; Carvalho, W.; Ferraz, A.; Milagres, A.M. Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol. Biofuels 2011, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, R.C. The Chemical Composition of Wood. In The Chemistry of Solid Wood; American Chemical Society: Washington, DC, USA, 1984; Volume 207, pp. 57–126. [Google Scholar]
- Kamthai, S.; Puthson, P. Effect of beating revolution on sweet bamboo Kraft pulp properties. CMU J. 2005, 4, 137–147. [Google Scholar]
- Baksi, S.; Saha, S.; Birgen, C.; Sarkar, U.; Preisig, H.A.; Markussen, S.; Wittgens, B.; Wentzel, A. Valorization of Lignocellulosic Waste (Crotalaria juncea) Using Alkaline Peroxide Pretreatment under Different Process Conditions: An Optimization Study on Separation of Lignin, Cellulose, and Hemicellulose. J. Nat. Fibers 2019, 16, 662–676. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. One-step bleaching process for cotton fabrics using activated hydrogen peroxide. Carbohydr. Polym. 2013, 92, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Zahran, M.K. One-step process for desizing, scouring and bleaching of cotton fabric using a novel ecofriendly bleaching agent. J. Text. Assoc. 2006, 67, 153–158. [Google Scholar]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef] [PubMed]
- El Ghzaour, A.; Trompette, J.L.; Cassanas, G.; Bardet, L.; Fabregue, E. Comparative rheological behavior of some cellulosic ether derivatives. Langmuir 2001, 17, 1453–1456. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Lopes, F.P.D.; Barbosa, A.P.; Bevitori, A.B.; Silva, I.L.A.D.; Costa, L.L.D. Natural Lignocellulosic Fibers as Engineering Materials—An Overview. Metall. Mater. Trans. A 2011, 42, 2963–2974. [Google Scholar] [CrossRef] [Green Version]
- Mohtar, S.S.; Tengku Malim Busu, T.N.Z.; Md Noor, A.M.; Shaari, N.; Mat, H. An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohydr. Polym. 2017, 166, 291–299. [Google Scholar] [CrossRef]
- Qi, H.; Liebert, T.; Meister, F.; Heinze, T. Homogenous carboxymethylation of cellulose in the NaOH/urea aqueous solution. React. Funct. Polym. 2009, 69, 779–784. [Google Scholar] [CrossRef]
- Motaung, T.E.; Mokhothu, T.H. The influence of supermasscolloider on the morphology of sugarcane bagasse and bagasse cellulose. Fibers Polym. 2016, 17, 343–348. [Google Scholar] [CrossRef]
- Alriols, M.G.; Tejado, A.; Blanco, M.; Mondragon, I.; Labidi, J. Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process. Chem. Eng. J. 2009, 148, 106–114. [Google Scholar] [CrossRef]
- Kamthai, S.; Magaraphan, R. Mechanical and barrier properties of spray dried carboxymethyl cellulose (CMC) film from bleached bagasse pulp. Ind. Crop. Prod. 2017, 109, 753–761. [Google Scholar] [CrossRef]






| Sample | Unbleached | Bleached with Various H2O2 Concentrations (v/v) | |||
|---|---|---|---|---|---|
| (0% H2O2) | 10 % | 20 % | 30 % | 40 % | |
| Cellulose-p |  |  |  |  |  |
| Code → | cellulose-p-0 | cellulose-p-10 | cellulose-p-20 | cellulose-p-30 | cellulose-p-40 |
| Cellulose-b |  |  |  |  |  |
| Code → | cellulose-b-0 | cellulose-b-10 | cellulose-b-20 | cellulose-b-30 | cellulose-b-40 |
| CMCp |  |  |  |  |  |
| Code → | CMCp-0 | CMCp-10 | CMCp-20 | CMCp-30 | CMCp-40 |
| CMCb |  |  |  |  |  |
| Code → | CMCb-0 | CMCb-10 | CMCb-20 | CMCb-30 | CMCb-40 |
| Film | H2O2 (% v/v) | Thickness (mm) | L* | WI | TS (MPa) | EB (%) | Solubility (%) | WVTR (g/d.m2) |
|---|---|---|---|---|---|---|---|---|
| CMCp | 0 | 0.213 ± 0.052 a | 50.3 ± 0.3 a | 44.9 ± 0.5 a | 5.68 ± 1.67 a | 56.18 ± 26.17 ab | 28.44 ± 5.51 a | 58.15 ± 4.45 a |
| 10 | 0.136 ± 0.006 bc | 60.2 ± 0.4 b | 47.6 ± 0.6 b | 7.77 ± 0.45 b | 68.73 ± 8.85 b | 34.85 ± 2.40 b | 58.23 ± 4.43 a | |
| 20 | 0.127 ± 0.011 b | 70.0 ± 0.7 d | 53.9 ± 0.9 c | 8.28 ± 1.06 b | 68.42 ± 7.57 b | 45.81 ± 1.36 c | 74.47 ± 8.93 b | |
| 30 | 0.144 ± 0.030 bc | 70.1 ± 0.4 d | 56.4 ± 0.6 d | 3.96 ± 1.14 c | 46.86 ± 14.44 a | 43.51 ± 3.78 c | 61.77 ± 7.24 a | |
| 40 | 0.156 ± 0.030 c | 68.9 ± 0.4 c | 57.1 ± 0.7 d | 1.41 ± 0.68 d | 42.86 ± 5.78 a | 36.70 ± 1.91 b | 55.92 ± 7.23 a | |
| CMCb | 0 | 0.111 ± 0.011 a | 85.3 ± 0.2 a | 81.5 ± 0.4 a | 13.94 ± 1.10 a | 79.08 ± 12.59 a | 36.12 ± 2.58 a | 46.28 ± 0.74 a |
| 10 | 0.083 ± 0.010 b | 86.2 ± 0.2 b | 84.6 ± 0.4 b | 16.91 ± 4.11 b | 86.64 ± 22.67 b | 37.70 ± 1.06 a | 51.72 ± 4.64 ab | |
| 20 | 0.081 ± 0.007 b | 86.2 ± 0.2 b | 84.1 ± 0.4 b | 29.09 ± 3.35 c | 84.92 ± 7.74 b | 66.65 ± 1.94 b | 62.94 ± 11.97 b | |
| 30 | 0.089 ± 0.014 b | 86.3 ± 0.4 b | 84.5 ± 0.3 b | 17.96 ± 2.68 b | 84.18 ± 11.10 b | 65.94 ± 3.99 b | 48.63 ± 11.13 ab | |
| 40 | 0.088 ± 0.008 b | 86.6 ± 0.1 b | 85.4 ± 0.8 c | 16.86 ± 3.03 b | 84.20 ± 11.41 b | 44.19 ± 1.28 c | 45.74 ± 1.94 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriyatem, R.; Noikang, N.; Kankam, T.; Jantanasakulwong, K.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Physical Properties of Carboxymethyl Cellulose from Palm Bunch and Bagasse Agricultural Wastes: Effect of Delignification with Hydrogen Peroxide. Polymers 2020, 12, 1505. https://doi.org/10.3390/polym12071505
Suriyatem R, Noikang N, Kankam T, Jantanasakulwong K, Leksawasdi N, Phimolsiripol Y, Insomphun C, Seesuriyachan P, Chaiyaso T, Jantrawut P, et al. Physical Properties of Carboxymethyl Cellulose from Palm Bunch and Bagasse Agricultural Wastes: Effect of Delignification with Hydrogen Peroxide. Polymers. 2020; 12(7):1505. https://doi.org/10.3390/polym12071505
Chicago/Turabian StyleSuriyatem, Rungsiri, Nichaya Noikang, Tamolwan Kankam, Kittisak Jantanasakulwong, Noppol Leksawasdi, Yuthana Phimolsiripol, Chayatip Insomphun, Phisit Seesuriyachan, Thanongsak Chaiyaso, Pensak Jantrawut, and et al. 2020. "Physical Properties of Carboxymethyl Cellulose from Palm Bunch and Bagasse Agricultural Wastes: Effect of Delignification with Hydrogen Peroxide" Polymers 12, no. 7: 1505. https://doi.org/10.3390/polym12071505