Bio-Composites Consisting of Cellulose Nanofibers and Na+ Montmorillonite Clay: Morphology and Performance Property
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Raw Materials and Sample Preparation
2.2. Characterization of CNFs and CNF/NC Composite Films
3. Results and Discussion
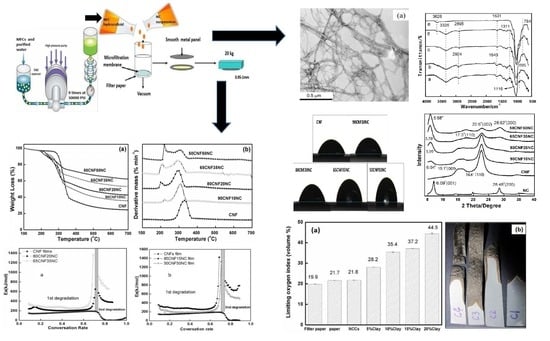
3.1. Morphology, Structure and Composition Characterization
3.2. Flame Retardant Properties of the CNF/NC Films
3.3. Wettability of the CNF/NC Composite Film
3.4. Porosity and Tensile Properties of the Composite Films
3.5. Thermal Degradation of the CNF/NC Composite Film
4. Conclusions
- 1)
- Better strength and elastic modulus were observed for films at low NC contents. Excess NC loading led to reduced strength properties due to NC aggregation in the film.
- 2)
- These composite films with about 35% NC demonstrated self-extinguishing ability when exposed to the open flame. Composites with over 35 wt % NC content did not burn because of the formation of a protective barrier containing ordered NC platelets.
- 3)
- The addition of montmorillonite NC increased the hydrophobicity of the material.
- 4)
- During the film thermal pyrolysis, the first process occurred between 100 and 200 °C, resulting mainly from evaporation of absorbed water; the second between 280 and 350 °C indicated thermal decomposition of cellulose; and the slow third stage happened from the 350 to 600 °C, representing carbonization. The results demonstrate that the apparent activation energies for all the CNF/NC composites were higher than the pure CNF film.
- 5)
- The CNF/NC films fabricated in this work seem promising as barrier materials for packaging applications.
Author Contributions
Funding
Conflicts of Interest
References
- Su, H.W.S.; Chen, W. High refractive index polyimide-nanocrystalline-titania hybrid optical materials. J. Mater. Chem. 2007, 18, 1139–1145. [Google Scholar] [CrossRef]
- Gabr, M.H.; Phong, N.T.; Abdelkareen, M.A.; Okubo, K.; Uzawa, K.; Isao, K.; Fuji, T. Mechanical, thermal, and moisture absorption properties of nano-clay reinforced nano-cellulose biocomposites. Cellulose 2013, 20, 819–826. [Google Scholar] [CrossRef]
- Cerruti, P.; Ambrogi, V.; Postiglione, A.; Rychly, J.; Matisova-Rychla, L.; Carfagna, C. Morphological and Thermal Properties of Cellulose-Montmorillonite Nanocomposites. Biomacromolecules 2008, 9, 3004–3013. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.D.; Walter, A.; Olli, I.; Lyuba, B.; Lars, A.B. Clay nanopaper with tough cellulose nanofiber matrix for fire retardancy and gas barrier functions. Biomacromolecules 2011, 12, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Yan, N. Hydrophobization of bleached softwood kraft fibers via adsorption of organo-nanoclay. Bioresource 2012, 7, 4132–4149. [Google Scholar]
- Sehaqui, H.; Liu, A.D.; Zhou, Q.; Berglund, L.A. Fast preparation procedure for large, flat cellulose and cellulose/inorganic nanopaper structures. Biomacromolecules 2010, 11, 2195–2198. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, H. Study on crystal structure of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzym. Microb. Technol. 2005, 36, 314–317. [Google Scholar] [CrossRef]
- Liu, A.D.; Lars, A.B. Fire-retardant and ductile clay nanopaper biocomposites based on montmorrilonite in matrix of cellulose nanofibers and carboxymethyl cellulose. Eur. Polym. J. 2013, 49, 940–949. [Google Scholar] [CrossRef]
- Walther, A.; Bjurhager, I.; Malho, J.M.; Pere, J.; Ruokolainen, J.; Lars, A.B.; Olli, I. Large-Area, Lightweight and Thick Biomimetic Composites with Superior Material Properties via Fast, Economic, and Green Pathways. Nano Lett. 2010, 8, 2742–2748. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Brown, M.E.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.L.; Kemmler, A.; et al. Computational aspects of kinetic analysis. Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.; Dubois, P. Polymer-layered NCte nano-composites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Henriksson, M.; Lars, A.B.; Isaksson, P.; Lindstrom, T.; Nishino, T. Cellulose Nanopaper Structures of High Toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Nayak, P.S.; Singh, B.K. Instrumental characterization of clay by XRF, XRD and FTIR. Bull. Mater. Sci. 2007, 30, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Lindstrom, T.; Banke, K.; Larsson, T.; Glad, N.G.; Boldizar, A. Nanoclay plating of cellulosic fiber surfaces. J. Apply Polym. Sci. 2008, 108, 887–891. [Google Scholar] [CrossRef]
- Han, J.Q.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-assembling behavior of cellulose nanoparticle during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hanna, M. Preparation and characterization of tapioca starch-poly (lactic acid)- Cloisite NA+ nano-composite foam. J. Appl. Polym. Sci. 2008, 110, 2337–2344. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.R.S.; Silva, R.C.; Silva, J.G.; Tonholo, J.; Ribeiro, A.S. Preparation and morphological characterization of chitosan/clay nano-composites. In Proceedings of the 11th International Conference on Advanced Materials, Rio de Janeiro, Brazil, 20–25 September2009; pp. 20–25. [Google Scholar]
- Ruiz-Hitzky, E.; Darder, M.; Aranda, P. An introdcution to bio-nanohybrid materials. In Bio-Inorganic Hybrid Nanomaterials: Strategies, Syntheses, Characterization and Applications; Rui-Hitzky, E., Ariga, K., Lvov, Y.M., Eds.; Wiley-VCH Verlag GmbH& CoKGaA: Weinheim, Germany, 2007; pp. 1–40. [Google Scholar]
- Samir, M.A.S.A.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nano-composite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.L.; Lei, Y.; Guo, W.H.; Xu, Y.J. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Bilbao, R.; Millera, A.; Arauzo, J. Thermal-decomposition of lignocellulosic materials e influence of the chemical-composition. Thermochim. Acta 1989, 143, 149–159. [Google Scholar] [CrossRef]
- Jakab, E.; Faix, O.; Till, F.; Szekely, T. Thermogravimetry mass-spectrometry study of 6 lignins within the scope of an international round-Robin test. J. Anal. Appl. Pyrolysis 1995, 35, 167–179. [Google Scholar] [CrossRef]
- Koufopanos, C.A.; Maschio, G.; Lucchesi, A. Kinetic modeling of the pyrolysis of biomass and biomass components. Can. J. Chem. Eng. 1989, 67, 75–84. [Google Scholar] [CrossRef]
- Gronli, M.G.; Varhegyi, G.; Di Blasi, C. Thermogravimetric analysis and devolatilization kinetics of wood. Ind. Eng. Chem. Res. 2002, 41, 4201–4208. [Google Scholar] [CrossRef]
- Varhegyi, G.; Antal, M.J.; Szekely, T.; Szabo, P. Kinetics of the thermaldecomposition of cellulose, hemicellulose, and sugar-cane bagasse. Energy Fuels 1989, 3, 329–335. [Google Scholar] [CrossRef]










| Method | Expression | Plots | Ref. |
|---|---|---|---|
| Kissinger | ln(β/T2p) = ln(AR/Ea) + (1/T)(−Ea/R) | ln(β/T2p) against 1/Tp | [11] |
| Coats–Redfern (modified) | log β = log (A Ea/Rg(α)) − 2.315 − 0.4567Ea/RT | logβ against 1/T | [12] |
| Flynn–Wall–Ozawa | ln(β/(T2(1 − 2RT/Ea ln(1 − α))) − Ea/RT | ln(β/T2) against 1/T | [13] |
| NC Content (wt%) | Density (kg/m3) | Porosity (%) | Tensile Strength (MPa) | Tensile Modulus (GPa) |
|---|---|---|---|---|
| 0 | 1305.04 | 13.00 | 121.0 ± 7.8 | 12.39 ± 0.83 |
| 10 | 1366.13 | 16.50 | 97.24 ± 3.85 | 9.30 ± 1.32 |
| 20 | 1386.56 | 21.75 | 69.48 ± 5.31 | 8.80 ± 1.15 |
| 35 | 1496.35 | 24.27 | 61.09 ± 5.97 | 6.02 ± 0.74 |
| 50 | 1589.21 | 27.10 | 53.77 ± 3.68 | 4.63 ± 0.83 |
| Sample | T01 a (°C) | WL01 (%) | T02 (°C) | WL02 (%) | Tp (°C) | WLp (%) | Ts (°C) | WLs (%) | Ts − T02 (°C) | WLs − WL02 (%) | Residue (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 90CNF10NC | 208 | 3.7 | 240 | 7.4 (0.4) | 288 | 20.2 (3.5) | 318 | 39.2 (2.0) | 78 | 31.9 | 36.2 |
| 80CNF20NC | 197 | 3.3 | 231 | 9.1 (0.3) | 281 | 19.6 (2.5) | 316 | 36.7 (1.1) | 85 | 27.6 | 42.9 |
| 65CNF35NC | 198 | 3.2 | 211 | 6.4 (0.6) | 281 | 19.6 (1.2) | 330 | 31.5 (0.1) | 119 | 25.1 | 53.1 |
| 50CNF50NC | 197 | 3.1 | 210 | 5.7 (0.2) | 278 | 19.3 (0.8) | 336 | 28.2 (0.1) | 126 | 22.5 | 62.2 |
| Sample | Kissinger | Coats–Redfern | Flynn–Wall–Ozawa | |||
|---|---|---|---|---|---|---|
| Ea | R2 | Ea | R2 | Ea | R2 | |
| CNF | 157.6 | 0.9862 | 181.6 (8.5) | 0.9904 | 182.2 (8.3) | 0.9914 |
| 90CNF/10NC | 186.6 | 0.9996 | 213.2 (13.0) | 0.9993 | 211.7 (12.6) | 0.9994 |
| 80CNF/20NC | 185.2 | 0.9881 | 215.6 (49.4) | 0.9934 | 213.8 (16.8) | 0.9940 |
| 65CNF/35NC | 206.1 | 0.9957 | 285.2 (48.4) | 0.9863 | 279.8 (12.8) | 0.9863 |
| 50CNF/50NC | 232.7 | 0.9352 | 284.5 (45.6) | 0.9849 | 289.7 (11.8) | 0.9836 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Zhang, X.; Li, H.; Zhou, D.; Wu, Q. Bio-Composites Consisting of Cellulose Nanofibers and Na+ Montmorillonite Clay: Morphology and Performance Property. Polymers 2020, 12, 1448. https://doi.org/10.3390/polym12071448
Huang R, Zhang X, Li H, Zhou D, Wu Q. Bio-Composites Consisting of Cellulose Nanofibers and Na+ Montmorillonite Clay: Morphology and Performance Property. Polymers. 2020; 12(7):1448. https://doi.org/10.3390/polym12071448
Chicago/Turabian StyleHuang, Runzhou, Xian Zhang, Huiyuan Li, Dingguo Zhou, and Qinglin Wu. 2020. "Bio-Composites Consisting of Cellulose Nanofibers and Na+ Montmorillonite Clay: Morphology and Performance Property" Polymers 12, no. 7: 1448. https://doi.org/10.3390/polym12071448