Development of Polyelectrolyte Complex Nanoparticles-PECNs Loaded with Ampicillin by Means of Polyelectrolyte Complexation and Ultra-High Pressure Homogenization (UHPH)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
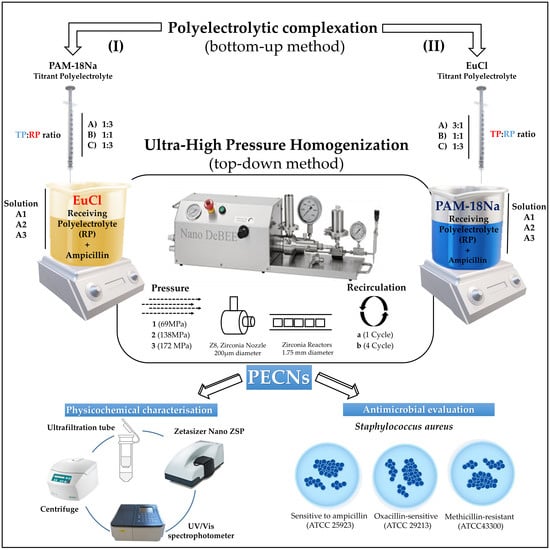
2.2. Preparation of Ampicillin-Loaded Polyelectrolyte Complex Nanoparticles
2.3. Physicochemical Characterization of Nanoparticles
2.4. Encapsulation Efficiency (EE)
2.5. Antimicrobial Effect of Nanoparticles
2.6. Statistical Analysis
3. Results and Discussion
3.1. Production and Characterization of Polyelectrolyte Complexes
3.1.1. Particle Size
3.1.2. PDI
3.1.3. Zeta Potential
3.2. Encapsulation Efficiency (EE)
3.3. Antimicrobial Effect of PECNs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zorraquín-Peña, I.; Cueva, C.; Bartolomé, B.; Moreno-Arribas, M.V. Silver nanoparticles against foodborne bacteria. Effects at intestinal level and health limitations. Microorganisms 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naddeo, J.J.; Ratti, M.; O’Malley, S.M.; Griepenburg, J.C.; Bubb, D.M.; Klein, E.A. Antibacterial Properties of Nanoparticles: A Comparative Review of Chemically Synthesized and Laser-Generated Particles. Adv. Sci. Eng. Med. 2015, 7, 1044–1057. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofac. Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Reiner, Ž.; Carbone, F.; Montecucco, F.; Sahebkar, A. The therapeutic potential of nanoparticles to reduce inflammation in atherosclerosis. Biomolecules 2019, 9, 416. [Google Scholar] [CrossRef] [Green Version]
- Fernando, S.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [Green Version]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Marassi, V.; Di Cristo, L.; Smith, S.G.J.; Ortelli, S.; Blosi, M.; Costa, A.L.; Reschiglian, P.; Volkov, Y.; Prina-Mello, A. Silver nanoparticles as a medical device in healthcare settings: A five-step approach for candidate screening of coating agents. R. Soc. Open Sci. 2018, 5, 171113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.E.; Jin, J.E.; Hwang, W.; Hong, S.W. Photocatalytic antibacterial application of zinc oxide nanoparticles and self-assembled networks under dual UV irradiation for enhanced disinfection. Int. J. Nanomed. 2019, 14, 1737–1751. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Nayak, T.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [Green Version]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.A.; Khlebtsov, N.G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiello, A.; et al. Metal-Based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity, and Their Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 3279–3300. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Salgado, A.J.; Sousa, N.; Mano, J.F.; Reis, R.L. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—A review. Prog. Polym. Sci. 2010, 35, 1163–1194. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.P.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconjug. Chem. 2015, 26, 1198–1211. [Google Scholar] [CrossRef]
- Trivedi, R.; Kompella, U.B. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ding, S.; Zhang, Z.; Wang, L.; You, Y. Cationic micelle: A promising nanocarrier for gene delivery with high transfection efficiency. J. Gene Med. 2019, 21, e3101. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Yang, Z.; Yao, L. Effectiveness of liposome bupivacaine for postoperative pain control in total knee arthroplasty: A PRISMA-compliant meta-analysis of randomized controlled trials. Medicine 2018, 97, e0171. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Moreno-Vega, A.I.; Gómez-Quintero, T.; Nuñez-Anita, R.E.; Acosta-Torres, L.S.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012, 936041. [Google Scholar] [CrossRef] [Green Version]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Valencia, G.A.; Zare, E.N.; Makvandi, P.; Gutiérrez, T.J. Self-Assembled Carbohydrate Polymers for Food Applications: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2009–2024. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Göktürk, E.; Erdal, H. Biomedical applications of polyglycolic acid (PGA). Sak. Üniversitesi Fen Bilim. Enstitüsü Derg. 2017, 21, 1237–1244. [Google Scholar] [CrossRef]
- Polyarylates for drug delivery and tissue engineering. Available online: https://patents.google.com/patent/US20050165203 (accessed on 1 April 2020).
- Salamanca, C.H.; Castillo, D.F.; Villada, J.D.; Rivera, G.R. Physicochemical characterization of in situ drug-polymer nanocomplex formed between zwitterionic drug and ionomeric material in aqueous solution. Mater. Sci. Eng. C 2017, 72, 405–414. [Google Scholar] [CrossRef]
- El-Nahas, A.E.; Allam, A.N.; Abdelmonsif, D.A.; El-Kamel, A.H. Silymarin-Loaded Eudragit Nanoparticles: Formulation, Characterization, and Hepatoprotective and Toxicity Evaluation. AAPS PharmSciTech 2017, 18, 3076–3086. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y.; Zhu, L.; Wang, B.; Guo, T. Preparation of Eudragit L 100-55 enteric nanoparticles by a novel emulsion diffusion method. Colloids Surf. B Biointerfaces 2013, 108, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.K.; El-Leithy, I.S.; Makky, A.A. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol. Pharm. 2010, 7, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, M.; Guo, X.; Zhou, Y. Multifunctional nanospheres embedded with quantum dots and magnetic nanoparticles for theranostic of prostatic cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1811. [Google Scholar] [CrossRef]
- Li, S.; Gu, F.; Gao, Q. Preparation of Rutin-Loaded Starch Nanospheres. Starch-Staerke 2018, 70, 1700116. [Google Scholar] [CrossRef]
- Jia, Y.; Fan, M.; Chen, H.; Miao, Y.; Xing, L.; Jiang, B.; Cheng, Q.; Liu, D.; Bao, W.; Qian, B.; et al. Magnetic hyaluronic acid nanospheres via aqueous Diels-Alder chemistry to deliver dexamethasone for adipose tissue engineering. J. Colloid Interface Sci. 2015, 15, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Shiv Shankar, S.; Bhargava, S.; Sastry, M. Synthesis of gold nanospheres and nanotriangles by the turkevich approach. J. Nanosci. Nanotechnol. 2005, 5, 1721–1727. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Calpena, A.C.; Folch, J.; Camins, A.; García, M.L. New potential strategies for Alzheimer’s disease prevention: Pegylated biodegradable dexibuprofen nanospheres administration to APPswe/PS1dE9. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1171–1182. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Meier, W. Polymer nanocapsules. Chem. Soc. Rev. 2000, 29, 295–303. [Google Scholar] [CrossRef]
- Yurgel, V.; Collares, T.; Seixas, F. Developments in the use of nanocapsules in oncology. Braz. J. Med. Biol. Res. 2013, 29, 295–303. [Google Scholar] [CrossRef]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The weapons for novel drug delivery systems. BioImpacts 2012, 2, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Mehra, N.K.; Jain, N.K. Potentials and emerging trends in nanopharmacology. Curr. Opin. Pharmacol. 2014, 15, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.M.; Vakili, M.R.; Molavi, O.; Lavasanifar, A. Self-Associating Poly(ethylene oxide)-block-poly(α-carboxyl-Îμ -caprolactone) Drug Conjugates for the Delivery of STAT3 Inhibitor JSI-124: Potential Application in Cancer Immunotherapy. Mol. Pharm. 2017, 8, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Cai, S.; Xie, Y.; Bagby, T.; Ren, S.; Forrest, M.L. Synthesis and characterization of a multiarm poly(acrylic acid) star polymer for application in sustained delivery of cisplatin and a nitric oxide prodrug. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2715–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, X.P.; Mai, T.T.T.; Ha, P.T.; Pham, H.N.; Luu, H.N.; Do, H.M.; Tran, D.L.; Nguyen, H.N.; Nguyen, L.T.; Ho, A.S.; et al. Multifunctional drug nanosystems: A summary of recent researches at IMS/VAST. In 5th International Conference on Biomedical Engineering in Vietnam; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Conejos-Sánchez, I.; Cardoso, I.; Oteo-Vives, M.; Romero-Sanz, E.; Paul, A.; Sauri, A.R.; Morcillo, M.A.; Saraiva, M.J.; Vicent, M.J. Polymer-doxycycline conjugates as fibril disrupters: An approach towards the treatment of a rare amyloidotic disease. J. Control. Release 2015, 28, 80–90. [Google Scholar] [CrossRef]
- Birch, N.P.; Schiffman, J.D. Characterization of self-Assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Zhu, Z.; Pu, K.Y.; Hu, Y.; Jiang, X.; Liu, B. Conjugated polyelectrolyte-cisplatin complex nanoparticles for simultaneous in vivo imaging and drug tracking. Nanoscale 2011, 3, 1997–2002. [Google Scholar] [CrossRef]
- Arora, S.; Gupta, S.; Narang, R.K.; Budhiraja, R.D. Amoxicillin loaded chitosan-alginate polyelectrolyte complex nanoparticles as mucopenetrating delivery system for H. pylori. Sci. Pharm. 2011, 79, 673–694. [Google Scholar] [CrossRef] [Green Version]
- Sarika, P.R.; James, N.R. Polyelectrolyte complex nanoparticles from cationised gelatin and sodium alginate for curcumin delivery. Carbohydr. Polym. 2016, 148, 354–361. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Monteiro, J.P.; Nocchi, S.R.; Silva, C.T.P.; Nakamura, C.V.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Preparation and cytotoxicity of N,N,N-trimethyl chitosan/alginate beads containing gold nanoparticles. Int. J. Biol. Macromol. 2015, 72, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte complexes: A review of their applicability in drug delivery technology. Indian J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taber, L.; Umerska, A. Polyelectrolyte complexes as nanoparticulate drug delivery systems. Eur. Pharm. Rev. 2015, 20, 36–40. [Google Scholar]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Dul, M.; Paluch, K.J.; Kelly, H.; Healy, A.M.; Sasse, A.; Tajber, L. Self-assembled carrageenan/protamine polyelectrolyte nanoplexes-Investigation of critical parameters governing their formation and characteristics. Carbohydr Polym. 2015, 123, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Garrigue, P.; Delville, M.H.; Labrugère, C.; Cloutet, E.; Kulesza, P.J.; Morand, J.P.; Kuhn, A. Top-down approach for the preparation of colloidal carbon nanoparticles. Chem. Mater. 2004, 16, 2984–2986. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.; Kannangara, Y.Y.; Lin, Y.; Amaratunga, G.A.J.; De Silva, K.M.N. A method for top down preparation of chitosan nanoparticles and nanofibers. Carbohydr. Polym. 2015, 117, 731–738. [Google Scholar] [CrossRef]
- Fu, X.; Cai, J.; Zhang, X.; Li, W.D.; Ge, H.; Hu, Y. Top-down fabrication of shape-controlled, monodisperse nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187. [Google Scholar] [CrossRef]
- Pereda, J.; Ferragut, V.; Quevedo, J.M.; Guamis, B.; Trujillo, A.J. Effects of Ultra-High Pressure Homogenization on Microbial and Physicochemical Shelf Life of Milk. J. Dairy Sci. 2010, 90, 1081–1093. [Google Scholar] [CrossRef]
- Briviba, K.; Gräf, V.; Walz, E.; Guamis, B.; Butz, P. Ultra high pressure homogenization of almond milk: Physico-chemical and physiological effects. Food Chem. 2016, 192, 82–89. [Google Scholar] [CrossRef]
- Zamora, A.; Guamis, B. Opportunities for Ultra-High-Pressure Homogenisation (UHPH) for the Food Industry. Food Eng. Rev. 2015, 7, 130–142. [Google Scholar] [CrossRef]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci. Technol. 2013, 31, 13–26. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Yarce, C.J.; Roman, Y.; Davalos, A.F.; Rivera, G.R. Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex. Pharmaceuticals 2018, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salamanca, C.H.; Yarce, C.J.; Zapata, C.A.; Giraldo, J.A. Relationship between the polymeric ionization degree and powder and surface properties in materials derived from poly(maleic anhydride-alt-octadecene). Molecules 2018, 23, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, V.; Yarce, C.J.; Echeverri, J.D.; Galeano, E.; Salamanca, C.H. Relationship between degree of polymeric ionisation and hydrolytic degradation of Eudragit® E polymers under extreme acid conditions. Polymers 2019, 11, 1010. [Google Scholar] [CrossRef] [Green Version]
- Malvern Panalytical. Dynamic Light Scattering: An Introduction in 30 min; Malvern Panalytical: Worcestershire, UK, 2011. [Google Scholar]
- M07-A10 CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard; tenth; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2015; Volume 35, pp. 1–87. [Google Scholar]
- Ciro, Y.; Rojas, J.; Oñate-Garzon, J.; Salamanca, C.H.; Ciro, Y.; Rojas, J.; Oñate-Garzon, J.; Salamanca, C.H. Synthesis, characterisation and biological evaluation of ampicillin-chitosan-polyanion nanoparticles produced by ionic gelation and polyelectrolyte complexation assisted by high-intensity sonication. Polymers 2019, 11, 1758. [Google Scholar] [CrossRef] [Green Version]
- Arévalo, L.M.; Yarce, C.J.; Oñate-Garzón, J.; Salamanca, C.H. Decrease of antimicrobial resistance through polyelectrolyte-coated nanoliposomes loaded with β-lactam drug. Pharmaceuticals 2019, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Alasino, R.V.; Ausar, S.F.; Bianco, I.D.; Castagna, L.F.; Contigiani, M.; Beltramo, D.M. Amphipathic and membrane-destabilizing properties of the cationic acrylate polymer Eudragit® E100. Macromol. Biosci. 2005, 15, 207–213. [Google Scholar] [CrossRef]
- Alasino, R.V.; Leonhard, V.; Bianco, I.D.; Beltramo, D.M. Eudragit E100 surface activity and lipid interactions. Colloids Surf. B Biointerfaces 2012, 91, 84–89. [Google Scholar] [CrossRef]
- Eswaranandam, S.; Hettiarachchy, N.S.; Johnson, M.G. Antimicrobial Activity of Citric, Lactic, Malic, or Tartaric Acids and Nisin-incorporated Soy Protein Film Against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J. Food Sci. 2006, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Liscano, Y.; Salamanca, C.H.; Vargas, L.; Cantor, S.; Laverde-Rojas, V.; Oñate-Garzón, J. Increases in hydrophilicity and charge on the polar face of alyteserin 1c helix change its selectivity towards gram-positive bacteria. Antibiotics 2019, 8, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, W.; Höltje, J.V. The architecture of the murein (peptidoglycan) in gram-negative bacteria: Vertical scaffold or horizontal layer(s)? J. Bacteriol. 2004, 186, 5978–5987. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Complexation Conditions | UHPH Conditions | Polyelectrolyte Complex Name | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case I | Case II | Titrant: Receiver Ratio | Pressure (MPa) | Cycles | ||||||
| PAM-18Na:EuCl | EuCl:PAM-18Na | A (1:3) | B (1:1) | C (3:1) | 1 (69) | 2 (138) | 3 (172) | a (1) | b (4) | |
| x | x | No UHPH | (control) | |||||||
| x | x | x | x | PECs-IA-1a | ||||||
| x | x | x | x | PECs-IA-1b | ||||||
| x | x | x | x | PECs-IA-2a | ||||||
| x | x | x | x | PECs-IA-2b | ||||||
| x | x | x | x | PECs-IA-3a | ||||||
| x | x | x | x | PECs-IA-3b | ||||||
| x | x | No UHPH | (control) | |||||||
| x | x | x | x | PECs-IB-1a | ||||||
| x | x | x | x | PECs-IB-1b | ||||||
| x | x | x | x | PECs-IB-2a | ||||||
| x | x | x | x | PECs-IB-2b | ||||||
| x | x | x | x | PECs-IB-3a | ||||||
| x | x | x | x | PECs-IB-3b | ||||||
| x | x | No UHPH | (control) | |||||||
| x | x | x | x | PECs-IC-1a | ||||||
| x | x | x | x | PECs-IC-1b | ||||||
| x | x | x | x | PECs-IC-2a | ||||||
| x | x | x | x | PECs-IC-2b | ||||||
| x | x | x | x | PECs-IC-3a | ||||||
| x | x | x | x | PECs-IC-3b | ||||||
| x | x | No UHPH | (control) | |||||||
| x | x | x | x | PECs-IIA-1a | ||||||
| x | x | x | x | PECs-IIA-1b | ||||||
| x | x | x | x | PECs-IIA-2a | ||||||
| x | x | x | x | PECs-IIA-2b | ||||||
| x | x | x | x | PECs-IIA-3a | ||||||
| x | x | x | x | PECs-IIA-3b | ||||||
| x | x | No UHPH | (control) | |||||||
| x | x | x | x | PECs-IIB-1a | ||||||
| x | x | x | x | PECs-IB-1b | ||||||
| x | x | x | x | PECs-IIB-2a | ||||||
| x | x | x | x | PECs-IIB-2b | ||||||
| x | x | x | x | PECs-IIB-3a | ||||||
| x | x | x | x | PECs-IIB-3b | ||||||
| x | x | No UHPH | (control) | |||||||
| x | x | x | x | PECs-IIC-1a | ||||||
| x | x | x | x | PECs-IIC-1b | ||||||
| x | x | x | x | PEC-IIC-2a | ||||||
| x | x | x | x | PEC-IIC-2b | ||||||
| x | x | x | x | PEC-IIC-3a | ||||||
| x | x | x | x | PEC-IIC-3b | ||||||
| Case | System | Molar Ratio Regarding Monomer Units | pH | |
|---|---|---|---|---|
| Titrant Polymer | Receiver Polymers | |||
| I | EuCl | 0 | 1 | 3.27 ± 0.01 |
| PECNs-IA | 1 | 3 | 4.85 ± 0.01 | |
| PECNs-IB | 1 | 1 | 8.75 ± 0.01 | |
| PECNs-IC | 3 | 1 | 9.65 ± 0.01 | |
| II | PAM-18Na | 0 | 1 | 10.84 ± 0.01 |
| PECNs-IIA | 1 | 3 | 9.80 ± 0.01 | |
| PECNs-IIB | 1 | 1 | 7.98 ± 0.01 | |
| PECNs-IIC | 3 | 1 | 4.70 ± 0.02 | |
| PECNs Family | Polyelectrolyte Complex | Particle Size (nm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| EuCl-PAM18Na (1:3 ratio) | PECN-IIA-1a | 130.1 ± 1.4 | 0.217 ± 0.011 | −56.6 ± 5.7 | 58.9 ± 2.0 |
| PECN-IIA-1b | 115.8 ± 1.3 | 0.204 ± 0.003 | −45.7 ± 0.7 | 54.8 ± 1.3 | |
| PECN-IIA-2a | 110.8 ± 1.1 | 0.194 ± 0.003 | −47.4 ± 2.8 | 56.3 ± 1.1 | |
| PECN-IIA-2b | 98.0 ± 0.9 | 0.189 ± 0.007 | −46.3 ± 2.1 | 53.2 ± 2.0 | |
| PECN-IIA-3a | 108.1 ± 0.9 | 0.215 ± 0.009 | −47.0 ± 4.4 | 56.3 ± 0.3 | |
| PECN-IIA-3b | 97.5 ± 1.0 | 0.194 ± 0.012 | −42.3 ± 1.3 | 50.0 ± 0.7 | |
| EuCl-PAM18Na (3:1 ratio) | PECN-IIC-1b | 140.1 ± 2.1 | 0.185 ± 0.024 | +43.9 ± 1.9 | 49.5 ± 1.4 |
| PECN-IIC-2a | 145.8 ± 3.5 | 0.242 ± 0.022 | +45.0 ± 2.6 | 46.1 ± 0.3 | |
| PECN-IIC-2b | 123.1 ± 0.9 | 0.194 ± 0.009 | +41.7 ± 1.4 | 45.1 ± 0.5 | |
| PECN-IIC-3a | 135.4 ± 2.2 | 0.187 ± 0.058 | +41.4 ± 1.6 | 43.5 ± 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, N.; Alhajj, M.J.; Sierra, M.; Oñate-Garzon, J.; Yarce, C.J.; Salamanca, C.H. Development of Polyelectrolyte Complex Nanoparticles-PECNs Loaded with Ampicillin by Means of Polyelectrolyte Complexation and Ultra-High Pressure Homogenization (UHPH). Polymers 2020, 12, 1168. https://doi.org/10.3390/polym12051168
Montero N, Alhajj MJ, Sierra M, Oñate-Garzon J, Yarce CJ, Salamanca CH. Development of Polyelectrolyte Complex Nanoparticles-PECNs Loaded with Ampicillin by Means of Polyelectrolyte Complexation and Ultra-High Pressure Homogenization (UHPH). Polymers. 2020; 12(5):1168. https://doi.org/10.3390/polym12051168
Chicago/Turabian StyleMontero, Nicolle, Maria J. Alhajj, Mariana Sierra, Jose Oñate-Garzon, Cristhian J. Yarce, and Constain H. Salamanca. 2020. "Development of Polyelectrolyte Complex Nanoparticles-PECNs Loaded with Ampicillin by Means of Polyelectrolyte Complexation and Ultra-High Pressure Homogenization (UHPH)" Polymers 12, no. 5: 1168. https://doi.org/10.3390/polym12051168