Forensic Engineering of Advanced Polymeric Materials—Part VII: Degradation of Biopolymer Welded Joints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
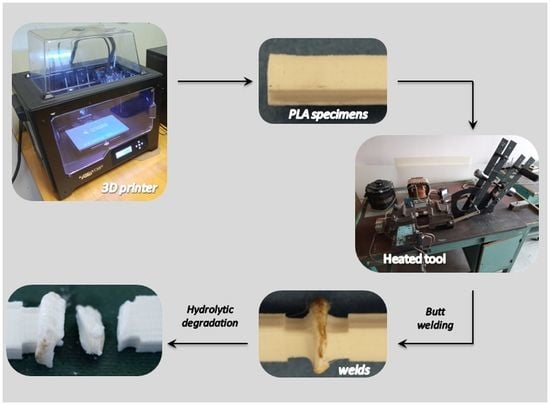
2.2. Description of The Products Samples Made of PLA and PHA Which Were Used to Make Welded Joints
2.3. Description of The Welded Joints and Method Used for Obtaining Thereof
2.4. Hydrolytic Degradation of Welded PLA and PHA Samples under Laboratory Conditions
2.5. Measurement Methods
2.5.1. Differential Scanning Calorimetry (DSC) Analysis
2.5.2. Determination of pH Changes
2.5.3. Gel Permeation Chromatography (GPC) Analysis
2.5.4. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
2.5.5. Electrospray Ionization Mass Spectrometry (ESI-MS) Analysis
2.5.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Structural Studies of The PLA and PHA Samples and Their Welds by 1H-NMR and GPC Analyses
3.2. Thermal Analysis of The Samples and Their Welds before The Hydrolytic Degradation Process
3.3. Hydrolytic Degradation Studies
3.4. Macroscopic Changes in The Samples Caused by Hydrolytic Degradation
3.5. Determination of The Chemical Structure of Hydrolytic Degradation Products of Biopolyester Welds by ESI-MS Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Sam, S.T.; Nuradibah, M.A.; Chin, K.M.; Hani, N. Current application and challenges on packaging industry based on natural polymer blending. In Natural Polymers: Industry Techniques and Applications; Olatunji, O., Ed.; Springer: Cham, Switzerland, 2016; pp. 163–185. [Google Scholar]
- Ahmed, J.; Varshney, S. Polylactides-chemistry, properties and green packaging technology: A review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Lemoigne, M. Products of dehydration and of polymerization of β-hydroxybutyric acid. Bull. Soc. Chem. Biol. 1926, 8, 770–782. [Google Scholar]
- Braunegg, G.; Lefebvre, G.; Genser, K.F. Polyhydroxyalkanoates, biopolyesters from renewable resources: Physiological and engineering aspects. J. Biotechnol. 1998, 65, 127–161. [Google Scholar] [CrossRef]
- Cacciotti, I.; Calderone, M.; Bianco, A. Tailoring the properties of electrospun PHBV mats: Co-solution blending and selective removal of PEO. Eur. Polym. J. 2013, 49, 3210–3222. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef] [Green Version]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Sikorska, W.; Musioł, M.; Zawidlak-Węgrzyńska, B.; Rydz, J. Compostable polymeric eco-materials: Environment-friendly waste management alternative to landfills. In Handbook of Eco-Materials; Torres-Martínez, L.M., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2018; 31p. [Google Scholar]
- Rydz, J.; Musioł, M.; Zawidlak-Węgrzyńska, B.; Sikorska, W. Present and future of (bio)degradable polymers for food packaging applications. In Handbook of Food Bioengineering; Grumezescu, A., Holban, A.M., Eds.; Academic Press: London, UK, 2018; Volume 20, pp. 429–465. [Google Scholar]
- Urbánek, T.; Jäger, E.; Jäger, A.; Hrubý, M. Selectively biodegradable polyesters: Nature-inspired construction materials for future biomedical applications. Polymers 2019, 11, 1061. [Google Scholar] [CrossRef] [Green Version]
- Michalak, M.; Kwiecień, I.; Kwiecień, M.; Adamus, G.; Odelius, K.; Hakkarainen, M.; Kurcok, P. Diversifying polyhydroxyalkanoates—End-group and side-chain functionality. Curr. Org. Synth. 2017, 14, 757–767. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- De Santis, M.; Cacciotti, I. Wireless implantable and biodegradable sensors for postsurgery monitoring: Current status and future perspectives. Nanotechnology 2020, 31, 252001. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, V.; Iurzhenko, M.; Shadrin, A.; Galchun, A. Relaxation behavior of polyethylene welded joints. Nanoscale Res. Lett. 2017, 12, 280–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galchun, A.; Korab, N.; Kondratenko, V.; Demchenko, V.; Shadrin, A.; Anistratenko, V.; Iurzhenko, M. Nanostructurization and thermal properties of polyethylenes’ welds. Nanoscale Res. Lett. 2015, 10, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinstein, L.; Frantz, J.; Grewell, D.; Lebron, K. Welding of PLA. In ANTEC® 2017: Proceeding of the Technical Conference and Exhibition, Anaheim, California, USA, 8–10 May 2017; Society of Plastics Engineers: Newtown, CT, USA, 2017; pp. 474–477. [Google Scholar]
- Pagano, N.; Campana, G.; Fiorini, M.; Morelli, R. Laser transmission welding of polylactide to aluminum thin films for applications in the food-packaging industry. Opt. Laser Technol. 2017, 91, 80–84. [Google Scholar] [CrossRef]
- Kowalczuk, M.M. Forensic engineering of advanced polymeric materials. Mathews J. Forensic Res. 2017, 1, e001. [Google Scholar]
- Monofinament Products. Available online: https://monofilament.com.ua/products/standartnye-materialy/pla/ (accessed on 25 December 2019).
- TianAn Home Page. Available online: www.tianan-enmat.com (accessed on 27 December 2019).
- Musioł, M.; Sikorska, W.; Adamus, G.; Kowalczuk, M. Forensic engineering of advanced polymeric materials. Part III-Biodegradation of thermoformed rigid PLA packaging under industrial composting conditions. Waste Manag. 2016, 52, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musioł, M.T.; Rydz, J.; Sikorska, W.J.; Rychter, P.R.; Kowalczuk, M.M. A preliminary study of the degradation of selected commercial packaging materials in compost and aqueous environments. Pol. J. Chem. Technol. 2011, 13, 55–57. [Google Scholar] [CrossRef] [Green Version]
- Rydz, J.; Sikorska, W.; Musioł, M.; Janeczek, H.; Włodarczyk, J.; Misiurska-Marczak, M.; Łęczycka, J.; Kowalczuk, M. 3D-Printed polyester-based prototypes for cosmetic applications—Future directions at the forensic engineering of advanced polymeric materials. Materials 2019, 12, 994. [Google Scholar] [CrossRef] [Green Version]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Muller, G.; Henning, S. Correlation between the degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Sikorska, W.; Musiol, M.; Nowak, B.; Pajak, J.; Labuzek, S.; Kowalczuk, M.; Adamus, G. Degradability of polylactide and its blend with poly[(R,S)-3-hydroxybutyrate] in industrial composting and compost extract. Int. Biodeter. Biodegr. 2015, 101, 32–41. [Google Scholar] [CrossRef]
- Sikorska, W.; Musioł, M.; Rydz, J.; Zięba, M.; Rychter, P.; Lewicka, K.; Šiškova, A.; Mosnácková, K.; Kowalczuk, M.; Adamus, G. Prediction studies of environment-friendly biodegradable polymeric packaging based on PLA. Influence of specimens’ thickness on the hydrolytic degradation profile. Waste Manag. 2018, 78, 938–947. [Google Scholar] [CrossRef]
- Rydz, J.; Wolna-Stypka, K.; Adamus, G.; Janeczek, H.; Musioł, M.; Sobota, M.; Marcinkowski, A.; Krzan, A.; Kowalczuk, M. Forensic engineering of advanced polymeric materials. Part 1—degradation studies of polylactide blends with atactic poly [(R,S)-3-hydroxybutyrate] in paraffin. Chem. Biochem. Eng. Q. 2015, 29, 247–259. [Google Scholar] [CrossRef]
- Adamus, G.; Sikorska, W.; Janeczek, H.; Kwiecień, M.; Sobota, M.; Kowalczuk, M. Novel block copolymers of atactic PHB with natural PHA for cardiovascular engineering: Synthesis and characterization. Eur. Polym. J. 2012, 48, 621–631. [Google Scholar] [CrossRef]
- Sikorska, W.; Richert, J.; Rydz, J.; Musioł, M.; Adamus, G.; Janeczek, H.; Kowalczuk, M. Degradability studies of poly(L-lactide) after multi-reprocessing experiments in extruder. Polym. Degrad. Stab. 2012, 97, 1891–1897. [Google Scholar] [CrossRef]
- Levy, A.; Le Corre, S.; Chevaugeon, N.; Poitou, A. A level set based approach for the finite element simulation of a forming process involving multiphysics coupling: Ultrasonic welding of thermoplastic composites. Eur. J. Mech. A Solid 2011, 30, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Musioł, M.; Sikorska, W.; Adamus, G.; Janeczek, H.; Kowalczuk, M.; Rydz, J. (Bio)degradable polymers as a potential material for food packaging: Studies on the (bio)degradation process of PLA/(R,S)-PHB rigid foils under industrial composting conditions. Eur. Food Res. Technol. 2016, 242, 815–823. [Google Scholar] [CrossRef]
- Rydz, J.; Adamus, G.; Wolna-Stypka, K.; Marcinkowski, A.; Misiurska-Marczak, M.; Kowalczuk, M.M. Degradation of polylactide in paraffin and selected protic media. Polym. Degrad. Stab. 2013, 98, 316–324. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Adamus, G.; Höglund, A.; Kowalczuk, M.; Albertsson, A.-C. ESI-MS reveals the influence of hydrophilicity and architecture on the water-soluble degradation product patterns of biodegradable homo- and copolyesters of 1,5-dioxepan-2-one and ε-caprolactone. Macromolecules 2008, 41, 3547–3554. [Google Scholar] [CrossRef]
- Höglund, A.; Hakkarainen, M.; Kowalczuk, M.; Adamus, G.; Albertsson, A.-C. Fingerprinting the degradation product patterns of different polyester-ether networks by Electrospray Ionization Mass Spectrometry. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4617. [Google Scholar] [CrossRef]
- Sikorska, W.; Adamus, G.; Dobrzynski, P.; Libera, M.; Rychter, P.; Krucinska, I.; Komisarczyk, A.; Cristea, M.; Kowalczuk, M. Forensic engineering of advanced polymeric materials—Part II: The effect of the solvent-free non-woven fabrics formation method on the release rate of lactic and glycolic acids from the tin-free poly(lactide-co-glycolide) nonwovens. Polym. Degrad. Stab. 2014, 110, 518–528. [Google Scholar] [CrossRef]
- Gonzalez Ausejo, J.; Rydz, J.; Musioł, M.; Sikorska, W.; Sobota, M.; Włodarczyk, J.; Adamus, G.; Janeczek, H.; Kwiecien, I.; Hercog, A.; et al. A comparative study of three-dimensional printing directions: The degradation and toxicological profile of a PLA/PHA blend. Polym. Degrad. Stab. 2018, 152, 191–207. [Google Scholar] [CrossRef] [Green Version]









| 3D-Printer Settings | |
|---|---|
| Extruder temperature, °C | 200 |
| Platform temperature, °C | 50 |
| Layer thickness, mm | 0.14 |
| First layer thickness, mm | 0.21 |
| Printing speed, mm/s | 80 |
| Moving speed, mm/s | 110 |
| Filling, % | 100 |
| Filling form | line |
| PLA Rigid Film | PLA 1 | 1W | PLA Filament | PLA 2 | 2W | ENMAT PHA | PHA 3 | 3W | |
|---|---|---|---|---|---|---|---|---|---|
| Heating Run of The Materials Tested | |||||||||
| Tg [°C] | 63.3 | n.d. | 63.7 ± 1.5 | 66.2 | 69.1 | 69.8 ± 2.4 | |||
| Tcc [°C] | 105.3 | 131.8 | 130.7 ± 1.3 | - | 110.9 | 109.5 ± 8.1 | - | - | - |
| ΔHcc [J/g] | 26.0 | 14.0 | 12.2 ± 0.9 | - | 18.6 | 22.4 ± 3.1 | - | - | - |
| Tm [°C] | 155.0 | 156.5 | 155.8 ± 1.4 | - | 171.5 | 175,1 ± 2.1 | 177.8 | 186.3 | 194.0 ± 4.9 |
| ΔHm [J/g] | 26.1 | 15.4 | 12.5 ± 1.2 | - | 19.7 | 23.7 ± 3.1 | 94.1 | 105.0 | 93.6 ± 5.4 |
| Xc [%] | - | - | - | - | - | - | 57.4 | 64.0 | 57.1 |
| Heating Run after Rapid Cooling from 200 °C | |||||||||
| Tg [°C] | 57.3 | 59.4 | 59.9 ± 0.1 | 62.0 | 63.6 | 62.8 ± 1.4 | 3.8 | 1.7 | −2.61 ± 3.7 |
| Tcc [°C] | 132.6 | 130.3 | 130.8 ± 0.3 | - | 110.9 | 106.9 ± 5.7 | 52.1 | 47.0 | 52.6 ± 0.8 |
| ΔHcc [J/g] | 2.5 | 8.8 | 8.8 ± 0.3 | - | 26.1 | 28.1 ± 1.5 | 96.4 | 98.3 | 77.8 ± 8.3 |
| Tm [°C] | 153.2 | 152.4 | 152.7 ± 0.2 | - | 171.7 | 170.4 ± 2.4 | 167.1 | 164.2 | 154.1 ± 6.8 |
| ΔHm [J/g] | 2.7 | 9.1 | 9.5 ± 0.3 | - | 27.0 | 28.5 ± 1.8 | 97.6 | 99.1 | 81.1 ± 4.6 |
| Xc [%] | - | - | - | - | - | - | 59.5 | 60.4 | 49.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska, W.; Zięba, M.; Musioł, M.; Kowalczuk, M.; Janeczek, H.; Chaber, P.; Masiuchok, O.; Demchenko, V.; Talanyuk, V.; Iurzhenko, M.; et al. Forensic Engineering of Advanced Polymeric Materials—Part VII: Degradation of Biopolymer Welded Joints. Polymers 2020, 12, 1167. https://doi.org/10.3390/polym12051167
Sikorska W, Zięba M, Musioł M, Kowalczuk M, Janeczek H, Chaber P, Masiuchok O, Demchenko V, Talanyuk V, Iurzhenko M, et al. Forensic Engineering of Advanced Polymeric Materials—Part VII: Degradation of Biopolymer Welded Joints. Polymers. 2020; 12(5):1167. https://doi.org/10.3390/polym12051167
Chicago/Turabian StyleSikorska, W., M. Zięba, M. Musioł, M. Kowalczuk, H. Janeczek, P. Chaber, O. Masiuchok, V. Demchenko, V. Talanyuk, M. Iurzhenko, and et al. 2020. "Forensic Engineering of Advanced Polymeric Materials—Part VII: Degradation of Biopolymer Welded Joints" Polymers 12, no. 5: 1167. https://doi.org/10.3390/polym12051167