Effect of Type and Concentration of Nanoclay on the Mechanical and Physicochemical Properties of Bis-GMA/TTEGDMA Dental Resins
Abstract
:1. Introduction
2. Materials and Methods
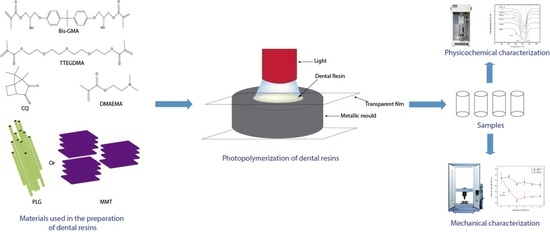
2.1. Materials
2.2. Preparation of Dental Composite
2.3. Characterization of Dental Composites
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Dynamic Mechanical Analysis (DMA)
2.3.4. Mechanical Properties
2.3.5. Depth of Cure
2.3.6. Sorption and Solubility
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Thermogravimetric Analysis (TGA)
3.3. Dynamic Mechanical Analysis (DMA)
3.4. Mechanical Properties
3.5. Depth of Cure
3.6. Sorption and Solubility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X. Inorganic Fillers for Dental Resin Composites: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. Recent Developments of New Components for Dental Adhesives and Composites. Macromol. Mater. Eng. 2007, 292, 245–271. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent Advances and Developments in Composite Dental Restorative Materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [Green Version]
- Alsharif, S.O.; Akil, H.B.M.; El-Aziz, N.A.; Ahmad, Z.A. Effect of alumina particles loading on the mechanical properties of light-cured dental resin composites. Mater. Des. 2014, 54, 430–435. [Google Scholar] [CrossRef]
- Campos, L.M.; Boaro, L.C.; Santos, T.M.; Marques, P.A.; Almeida, S.R.; Braga, R.R.; Parra, D.F. Evaluation of flexural modulus, flexural strength and degree of conversion in BISGMA/TEGDMA resin filled with montmorillonite nanoparticles. J. Compos. Mater. 2017, 51, 927–937. [Google Scholar] [CrossRef]
- Discacciati, J.A.; Oréfice, R.L. Structural analysis on photopolymerized dental resins containing nanocomponents. J. Mater. Sci. 2007, 42, 3883–3893. [Google Scholar] [CrossRef]
- Mahmoodian, M.; Pourabbas, B.; Arya, A.B. Preparation and Characterization of Bis-GMA/TEGDMA/Clay Nanocomposites at Low Filler Content Regimes. J. Compos. Mater. 2010, 44, 1379–1395. [Google Scholar] [CrossRef]
- Mucci, V.; Pérez, J.; Vallo, C.I. Preparation and characterization of light-cured methacrylate/montmorillonite nanocomposites. Polym. Int. 2010, 60, 247–254. [Google Scholar] [CrossRef]
- Terrin, M.M.; Poli, A.L.; Horn, M.A.; Neumann, M.G.; Cavalheiro, E.T.; Correa, I.C.; Schmitt, C.C. Effect of the loading of organomodified clays on the thermal and mechanical properties of a model dental resin. Mat. Res. 2016, 19, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Campos, L.M.; Boaro, L.C.; Ferreira, H.P.; Gomes dos Santos, L.K.; Ribeiro dos Santos, T.; Parra, D.F. Evaluation of polymerization shrinkage in dental restorative experimental composites based: BisGMA/TEGDMA, filled with MMT. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Campos, L.M.; Lugao, A.B.; Vasconcelos, M.R.; Parra, D.F. Polymerization Shrinkage Evaluation on Nanoscale-Layered Silicates: Bis-GMA/TEGMA Nanocomposites, in Photo-Activated Polymeric Matrices. J. Appl. Polym. Sci. 2014, 131, 1–6. [Google Scholar]
- Tian, M.; Gao, Y.; Liu, Y.; Liao, Y.; Hedin, N.E.; Fong, H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing nano fibrillar silicate. Dent. Mater. 2008, 24, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Gao, Y.; Chen, Q.; Tian, M.; Fong, H. Bis-GMA/TEGDMA dental composites reinforced with nano-scaled single crystals of fibrillar silicate. J. Mater. Sci. 2010, 45, 2521–2524. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Leitune, V.C.B.; Takimi, A.S.; Collares, F.M.; Sauro, S. Physicochemical and bioactive properties of innovative resin-based materials containing functional halloysite-nanotubes fillers. Dent. Mater. 2016, 32, 1133–1143. [Google Scholar] [CrossRef]
- Rüttermann, S.; Dluzhevskaya, I.; Großsteinbeck, C.; Raab, W.H.; Janda, R. Impact of replacing Bis-GMA and TEGDMA by other commercially available monomers on the properties of resin-based composites. Dent. Mater. 2010, 26, 353–359. [Google Scholar] [CrossRef]
- Indrani, D.J.; Cook, W.D.; Televantos, F.; Tyas, M.J.; Harcourt, J.K. Fracture toughness of water-aged resin composite restorative materials. Dent. Mater. 1995, 11, 201–207. [Google Scholar] [CrossRef]
- ISO 4049:2009 Dentistry—Polymer-Based Restorative Materials; ISO: Geneva, Switzerland, 2009.
- ASTM D 695—02a Standard Test Method for Compressive Properties of Rigid Plastic; ASTM International: West Conshohocken, PA, USA, 2002.
- Thorat, S.; Patra, N.; Ruffilli, R.; Diaspro, A.; Salerno, M. Preparation and characterization of a BisGMA-resin dental restorative composites with glass, silica and titania fillers. Dent. Mater. J. 2012, 31, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.; Samuel, S.M. Discrepancies in degree of conversión measurements by FTIR. Braz. Oral Res. 2013, 28, 453–454. [Google Scholar]
- Teshima, W.; Nomura, Y.; Ikeda, A.; Kawahara, T.; Okazaki, M.; Nahara, Y. Thermal degradation of photo-polymerized BisGMA/TEGDMA-based dental resins. Polym. Degrad. Stab. 2004, 84, 167–172. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Garfias-Mesías, L.F.; Paul, D.R. Thermal degradation of commercially available organoclays studied by TGA–FTIR. Thermochima Acta 2007, 457, 92–102. [Google Scholar] [CrossRef]
- Munhoz, T.; Fredholm, Y.; Rivory, P.; Balvay, S.; Hartmann, D.; Silva, P.; Chenal, J.M. Effect of nanoclay addition on physical, chemical, optical and biological properties of experimental dental resin composites. Dent. Mater. 2017, 33, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.R.; Silva, E.O. The Use of Montmorillonite Clays as Reinforcing Fillers for Dental Adhesives. Mat. Res. 2016, 19, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Menezes, L.R.; Silva, E.O.; Rocha, A.C.; Oliveira, D.C.; Campos, P.R. The applicability of organomodified nanoclays as new fillers for mechanical reinforcement of dental composites. J. Compos. Mater. 2018, 52, 963–970. [Google Scholar] [CrossRef]
- Aydınoğlu, A.; Yoruç, A.B. Effects of silane-modified fillers on properties of dental composite resin. Mater. Sci. Eng. C 2017, 79, 382–389. [Google Scholar] [CrossRef] [PubMed]







| Nanoclay Content (wt.%) | Composites | |
|---|---|---|
| MMT | PLG | |
| 0 | Unfilled | |
| 2 | MMT-2 | PLG-2 |
| 4 | MMT-4 | PLG-4 |
| 6 | MMT-6 | PLG-6 |
| 8 | MMT-8 | PLG-8 |
| 10 | MMT-10 | PLG-10 |
| Nanoclay Content (%) | Tg (°C) | |
|---|---|---|
| MMT | PLG | |
| 0 | 110 | |
| 2 | 108 | 111 |
| 4 | 110 | 112 |
| 6 | 110 | 116 |
| 8 | 112 | 116 |
| 10 | 112 | 116 |
| Nanoclay Content (%) | Depth of cure (mm) | |
|---|---|---|
| MMT | PLG | |
| 0 | 3.0 + 0.01 | |
| 2 | 2.99 + 0.01 | 2.99 + 0.02 |
| 4 | 2.99 + 0.01 | 2.99 + 0.01 |
| 6 | 2.99 + 0.02 | 2.99 + 0.02 |
| 8 | 2.99 + 0.03 | 2.99 + 0.02 |
| 10 | 2.99 + 0.04 | 2.99 + 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encalada-Alayola, J.J.; Veranes-Pantoja, Y.; Uribe-Calderón, J.A.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Effect of Type and Concentration of Nanoclay on the Mechanical and Physicochemical Properties of Bis-GMA/TTEGDMA Dental Resins. Polymers 2020, 12, 601. https://doi.org/10.3390/polym12030601
Encalada-Alayola JJ, Veranes-Pantoja Y, Uribe-Calderón JA, Cauich-Rodríguez JV, Cervantes-Uc JM. Effect of Type and Concentration of Nanoclay on the Mechanical and Physicochemical Properties of Bis-GMA/TTEGDMA Dental Resins. Polymers. 2020; 12(3):601. https://doi.org/10.3390/polym12030601
Chicago/Turabian StyleEncalada-Alayola, J. J., Y. Veranes-Pantoja, J. A. Uribe-Calderón, J. V. Cauich-Rodríguez, and J. M. Cervantes-Uc. 2020. "Effect of Type and Concentration of Nanoclay on the Mechanical and Physicochemical Properties of Bis-GMA/TTEGDMA Dental Resins" Polymers 12, no. 3: 601. https://doi.org/10.3390/polym12030601