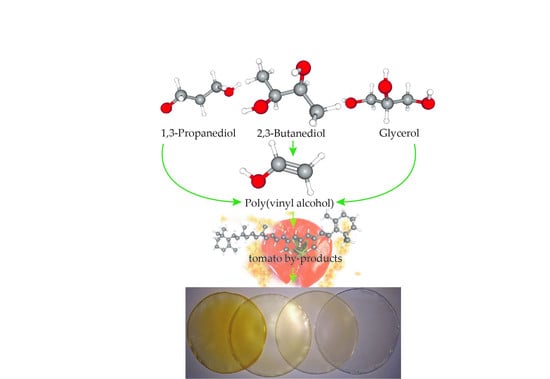
Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bioactive Compound Extraction from Tomato By-Products
2.2.1. Tomato Pigments Extracted by Ultrasound
2.2.2. HPLC Analysis of Carotenoid Pigments
2.3. PVOH-Based Biofilm Preparation
2.4. Flow Behavior Measurements of Biofilm Solution
2.5. Characterization of the Solid Biofilms
2.5.1. Physical Measurements
2.5.2. Biofilms FTIR Analysis
2.5.3. Biofilms UV–Vis Analysis
2.5.4. Biofilms Mechanical Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Carotenoid Pigments Extracted from Tomato By-Products
3.2. Flow Behavior of the PVOH-Based Biofilm Solutions
3.3. Solid Biofilm Characterization
3.3.1. Physical Appearance and Measurements
3.3.2. FTIR Analysis
3.3.3. UV–Vis Analysis
3.3.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cherubini, F.; Ulgiati, S. Crop residues as raw materials for biorefinery systems—A LCA case study. Appl. Energy 2010, 87, 47–57. [Google Scholar] [CrossRef]
- Kourmentza, C.; Placido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, M.; Marsalek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. N. Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the plastic crisis: A review throughout the plastic life cycle. Wires Energy Environ. 2019, 9, e360. [Google Scholar] [CrossRef] [Green Version]
- Raddadi, N.; Fava, F. Biodegradation of oil-based plastics in the environment: Existing knowledge and needs of research and innovation. Sci. Total Environ. 2019, 679, 148–158. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Wantzen, K.M. Threats Underestimated in Freshwater Plastic Pollution: Mini-Review. Water Air Soil Poll. 2019, 230. [Google Scholar] [CrossRef]
- Wang, M.H.; He, Y.; Sen, B. Research and management of plastic pollution in coastal environments of China. Environ. Pollut. (Barking Essex: 1987) 2019, 248, 898–905. [Google Scholar] [CrossRef]
- Hillmyer, M.A. The promise of plastics from plants. Science 2017, 358, 868–870. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Innovating for Sustainable Growth. In A Bioeconomy for Europe; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Carus, M.; Dammer, L. The Circular Bioeconomy—Concepts, Opportunities, and Limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- Xu, A.R.; Chen, L.; Wang, J.J. Functionalized Imidazalium Carboxylates for Enhancing Practical Applicability in Cellulose Processing. Macromolecules 2018, 51, 4158–4166. [Google Scholar] [CrossRef]
- Popescu, R.A.; Magyari, K.; Vulpoi, A.; Trandafir, D.L.; Licarete, E.; Todea, M.; Stefan, R.; Voica, C.; Vodnar, D.C.; Simon, S.; et al. Bioactive and biocompatible copper containing glass-ceramics with remarkable antibacterial properties and high cell viability designed for future in vivo trials. Biomater. Sci. 2016, 4, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Rapa, M.; Stefan, M.; Stan, M.; Macavei, S.; Darie-Nita, R.N.; Barbu-Tudoran, L.; Vodnar, D.C.; Popa, E.E.; Stefan, R.; et al. New PLA/ZnO:Cu/Ag bionanocomposites for food packaging. Express Polym. Lett. 2017, 11, 531–544. [Google Scholar] [CrossRef]
- Xu, A.R.; Wang, Y.X.; Gao, J.Y.; Wang, J.J. Facile fabrication of a homogeneous cellulose/polylactic acid composite film with improved biocompatibility, biodegradability and mechanical properties. Green Chem. 2019, 21, 4449–4456. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- Assis, R.Q.; Pagno, C.H.; Costa, T.M.H.; Flôres, S.H.; Rios, A.d.O. Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β-carotene. Packag. Technol. Sci. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Precup, G.; Calinoiu, L.F.; Mitrea, L.; Bindea, M.; Rusu, B.; Stefanescu, B.E.; Vodnar, D.C. The Molecular Restructuring of Classical Desserts by Using Food Industry By-Products. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2017, 74, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Calinoiu, L.F.; Stefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Latos-Brozio, M.; Masek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 110975. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Calinoiu, L.F.; Vodnar, D.C. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends in Food Science & Technology 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Assis, R.Q.; Rios, P.D.A.; Rios, A.d.O.; Olivera, F.C. Biodegradable packaging of cellulose acetate incorporated with norbixin, lycopene or zeaxanthin. Ind. Crops Prod. 2020, 147, 112212. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Catoi, A.F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanatt, S.R.; Makwana, S.H. Development of active, water-resistant carboxymethyl cellulose-poly vinyl alcohol-Aloe vera packaging film. Carbohydr. Polym. 2020, 227, 115303. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R.; Konieczny, K. Robust poly (vinyl alcohol) membranes containing chitosan/chitosan derivatives microparticles for pervaporative dehydration of ethanol. Sep. Purif. Technol. 2020, 234, 116094. [Google Scholar] [CrossRef]
- Xia, L.L.; Li, C.L.; Wang, Y. In-situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Membr. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Ji, C.H.; Xu, X.R.; Ma, X.H.; Xu, Z.L. Multilayer assembled CS-PSS/ceramic hollow fiber membranes for pervaporation dehydration. Sep. Purif. Technol. 2018, 203, 84–92. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Q.; Wu, C.L.; Wang, H.Y.; Wang, H. Viscosity-driven in situ self-assembly strategy to fabricate cross-linked ZIF-90/PVA hybrid membranes for ethanol dehydration via pervaporation. Sep. Purif. Technol. 2018, 201, 256–267. [Google Scholar] [CrossRef]
- Flynn, E.J.; Keane, D.A.; Tabari, P.M.; Morris, M.A. Pervaporation performance enhancement through the incorporation of mesoporous silica spheres into PVA membranes. Sep. Purif. Technol. 2013, 118, 73–80. [Google Scholar] [CrossRef]
- Saxena, S.K. Polyvinyl Alcohol (PVA) Chemical and Technical Assessment (CTA). Chemical and Technical Assessment—FAO. Available online: https://scholar.google.com/scholar_lookup?author=S.+K.+Saxena+&publication_year=2004&title=Polyvinyl+Alcohol+(PVA)+Chemical+and+Technical+Assessment+(CTA) (accessed on 5 December 2004).
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2020, 60, 171–202. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.E.; Mitrea, L.; Călinoiu, L.F.; Martau, G.A.; Simon EVarvara, R.A.; Vodnar, D.C. Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Della Pina, C.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Shukla, K.; Srivastava, V.C. Synthesis of organic carbonates from alcoholysis of urea: A review. Catal. Rev. Sci. Eng. 2017, 59, 1–43. [Google Scholar] [CrossRef]
- Mitrea, L.; Calinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Stefanescu, B.E.; Pop, I.D.; Vodnar, D.C. Isolated Microorganisms for Bioconversion of Biodiesel-Derived Glycerol Into 1,3-Propanediol. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.S.; Aroua, M.K.; Daud, W.M.A.W.; Cognet, P.; Peres-Lucchese, Y.; Fabre, P.L.; Reynes, O.; Latapie, L. A review: Conversion of bioglycerol into 1,3-propanediol via biological and chemical method. Renew. Sustain. Energy Rev. 2015, 42, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Samudrala, P.S. Glycerol Transformation to Value-Added 1,3-Propanediol Production: A Paradigm for a Sustainable Biorefinery Process. In Glycerine Production and Transformation—An Innovative Platform for Sustainable Biorefinery and Energy; IntechOpen: London, UK, 2019. [Google Scholar]
- Shrivastav, A.; Lee, J.; Kim, H.Y.; Kim, Y.R. Recent insights in the removal of Klebsiella pathogenicity factors for the industrial production of 2,3-butanediol. J. Microbiol. Biotechnol. 2013, 23, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Bialkowska, A.M. Strategies for efficient and economical 2,3-butanediol production: New trends in this field. World J. Microbiol. Biotechnol. 2016, 32, 200. [Google Scholar] [CrossRef]
- Mitrea, L.; Trif, M.; Catoi, A.F.; Vodnar, D.C. Utilization of biodiesel derived-glycerol for 1,3-PD and citric acid production. Microb. Cell Fact. 2017, 16, 190. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Calinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.J.; Stefanescu, B.E.; Vodnar, D.C. Inhibitory Potential Of Lactobacillus Plantarum on Escherichia Coli. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2017, 74, 99–101. [Google Scholar] [CrossRef] [Green Version]
- Calinoiu, L.F.; Vodnar, D.; Precup, G. A Review: The Probiotic Bacteria Viability under Different Conditions. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73. [Google Scholar] [CrossRef] [Green Version]
- Celinska, E.; Grajek, W. Biotechnological production of 2,3-butanediol--current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Diaconeasa, Z.; Catoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre-Loredo, R.Y.; Rodriguez-Hernandez, A.I.; Morales-Sanchez, E.; Gomez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.S.N.; Ali, R.R.; Zainal, M.F.H. Thermal Properties and Moisture Content Analysis of Starch Fiber Filled Polyvinyl Alcohol-Polyvinyl Acetate (Pva-Pvac) Biofilm. Int. J. Eng. Technol. 2018, 7, 81–83. [Google Scholar] [CrossRef]
- Murrieta-Martinez, C.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodriguez-Felix, F.; Ramirez-Wong, B.; Santacruz-Ortega, H.; Santos-Sauceda, I.; Olibarria-Rodriguez, G.; Marquez-Rios, E. Effect of Different Polyalcohols as Plasticizers on the Functional Properties of Squid Protein Film (Dosidicus Gigas). Coatings 2019, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Chen, S.C.; Wang, X.L.; Wang, Y.Z. Preparation and Rheological Behaviors of Thermoplastic Poly (vinyl alcohol) Modified by Lactic Acid. Ind. Eng. Chem. Res. 2011, 50, 9123–9130. [Google Scholar] [CrossRef]
- Shi, S.J.; Peng, X.; Liu, T.Q.; Chen, Y.N.; He, C.C.; Wang, H.L. Facile preparation of hydrogen-bonded supramolecular polyvinyl alcohol-glycerol gels with excellent thermoplasticity and mechanical properties. Polymer 2017, 111, 168–176. [Google Scholar] [CrossRef]
- Mojarad-Jabali, S.; Kabiri, K.; Karami, Z.; Mastropietro, D.J.; Omidian, H. Surface cross-linked SAPs with improved swollen gel strength using diol compounds. J. Macromol. Sci. Part A-Pure Appl. Chem. 2020, 57, 62–71. [Google Scholar] [CrossRef]
- Muresan, V.; Danthine, S.; Racolta, E.; Muste, S.; Blecker, C. The Influence of Particle Size Distribution on Sunflower Tahini Rheology and Structure. J. Food Process. Eng. 2014, 37, 411–426. [Google Scholar] [CrossRef]
- Sukhlaaied, W.; Riyajan, S.A.; Palmese, G.R. Dynamic viscosity of maleate poly (vinyl alcohol) and its copolymer measured by rheometer. Polym. Test. 2016, 56, 387–393. [Google Scholar] [CrossRef]
- Tian, H.F.; Yuan, L.; Zhou, D.; Niu, J.Y.; Cong, H.J.; Xiang, A.M. Improved mechanical properties of poly (vinyl alcohol) films with glycerol plasticizer by uniaxial drawing. Polym. Adv. Technol. 2018, 29, 2612–2618. [Google Scholar] [CrossRef]
- Boonsuk, P.; Sukolrat, A.; Kaewtatip, K.; Chantarak, S.; Kelarakis, A.; Chaibundit, C. Modified cassava starch/poly (vinyl alcohol) blend films plasticized by glycerol: Structure and properties. J. Appl. Polym. Sci. 2020. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Zhang, F.-C.; Qu, D.-Z.; Bai, Y.-P. The effect of crystallization properties influenced by 2-methyl-1,3–propanediol units on the optical properties of modified poly (ethylene terephthalate). High Perform. Polym. 2018, 31, 211–219. [Google Scholar] [CrossRef]
- Sand, A.; Kniivilä, J.; Toivakka, M.; Hjelt, T. Structure formation mechanisms in consolidating pigment coatings—Simulation and visualisation. Chem. Eng. Process. Process Intensif. 2011, 50, 574–582. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res 2019. [Google Scholar]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT-Food Sci. Technol. 2019, 116, 108558. [Google Scholar] [CrossRef]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; Kerry, J.P. Effect of lutein, sesamol, ellagic acid and olive leaf extract on the quality and shelf-life stability of packaged raw minced beef patties. Meat Sci. 2010, 84, 613–620. [Google Scholar] [CrossRef]
- Phoopuritham, P.; Thongngam, M.; Yoksan, R.; Suppakul, P. Antioxidant Properties of Selected Plant Extracts and Application in Packaging as Antioxidant Cellulose-Based Films for Vegetable Oil. Packag. Technol. Sci. 2012, 25, 125–136. [Google Scholar] [CrossRef]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focsan, M.; Rugina, D.O.; Pintea, A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and pH-sensitive films developed by incorporating purple and black rice extracts into chitosan matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef]
- Wu, Y.; Ying, Y.; Liu, Y.; Zhang, H.; Huang, J. Preparation of chitosan/poly vinyl alcohol films and their inhibition of biofilm formation against Pseudomonas aeruginosa PAO1. Int. J. Biol. Macromol. 2018, 118, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.-Y.; Bo, C.-Y.; Hu, L.-H.; Zhou, Y.-H. Properties of Poly (vinyl alcohol) Plasticized by Glycerin. J. For. Prod. Ind. 2014, 2, 151–153. [Google Scholar]
- Bhatia, S.K.; Kurian, J.V. Biological characterization of Sorona polymer from corn-derived 1,3-propanediol. Biotechnol. Lett. 2008, 30, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, K.; Martin, L.; Gonzalez, A.; Retegi, A.; Eceiza, A.; Gabilondo, N. D-isosorbide and 1,3-propanediol as plasticizers for starch-based films: Characterization and aging study. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Mitrea, L.; Vodnar, D.C. Klebsiella pneumoniae-A Useful Pathogenic Strain for Biotechnological Purposes: Diols Biosynthesis under Controlled and Uncontrolled pH Levels. Pathogens 2019, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.V.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms 2019, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wang, Y.; Xu, J.; Ahmed, S.; Liu, Y.W. Preparation and Characterization of Ultrasound Treated Polyvinyl Alcohol/Chitosan/DMC Antimicrobial Films. Coatings 2019, 9, 582. [Google Scholar] [CrossRef] [Green Version]
- Harun-or-Rashid, M.D.; Rahaman, M.D.S.; Kabir, S.E.; Khan, M.A. Effect of hydrochloric acid on the properties of biodegradable packaging materials of carboxymethylcellulose/poly (vinyl alcohol) blends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Yu, Z.; Alsammarraie, F.K.; Nayigiziki, F.X.; Wang, W.; Vardhanabhuti, B.; Mustapha, A.; Lin, M. Effect and mechanism of cellulose nanofibrils on the active functions of biopolymer-based nanocomposite films. Food Res. Int. 2017, 99, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, N.B.; Kljajevic, L.M.; Popovic, I.G.; Filipovic, J.M.; Krusic, M.T.K. Chitosan, itaconic acid and poly (vinyl alcohol) hybrid polymer networks of high degree of swelling and good mechanical strength. Polym. Int. 2010, 59, 686–694. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranska, M.; Schutze, W.; Schulz, H. Determination of lycopene and beta-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Ihl, M.; Bifani, V.; Silva, A.; Montero, P. Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz). Food Hydrocoll. 2007, 21, 1133–1143. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Emiroglu, Z.K.; Yemis, G.P.; Coskun, B.K.; Candogan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Q.; Ma, T.; Bao, K.; Yu, X.; Duan, Z.; Shen, X.; Li, C. Effect of EGCG-gelatin biofilm on the quality and microbial composition of tilapia fillets during chilled storage. Food Chem. 2020, 305, 125454. [Google Scholar] [CrossRef]
- Chan, K.S.; Senin, H.B.; Naimah, I. Structural and Mechanical Properties of Polyvinyl Alcohol (Pva) Thin Film. Nanosci. Nanotechnol. 2009, 1136, 366–369. [Google Scholar] [CrossRef]









| Peak No. | Compound | Rt (min) | λmax (nm) | Q (mg/100 DW) |
|---|---|---|---|---|
| 1 | Lutein | 6.41 | 448, 474 | 1.498 ± 0.06 |
| 2 | Lycopene | 13.42 | 446, 473 | 0.135 ± 0.01 |
| 3 | β-carotene | 14.51 | 455, 480 | 1.605 ± 0.05 |
| PVOH Formulations | Moisture Content (%) |
|---|---|
| PVOH | 3.75 ± 0.00 |
| PVOH+Gly | 26.91 ± 0.04 |
| PVOH+PDO | 5.59 ± 0.01 |
| PVOH+BDO | 8.04 ± 0.03 |
| PVOH+TP | 3.73 ± 0.00 |
| PVOH+Gly+TP | 11.12 ± 0.01 |
| PVOH+PDO+TP | 4.76 ± 0.01 |
| PVOH+BDO+TP | 6.44 ± 0.02 |
| Polymeric Films | Transparency (nm/mm) |
|---|---|
| PVOH | 0.13 ± 0.02 e |
| PVOH+Gly | 1.10 ± 0.04 c,d |
| PVOH+PDO | 0.76 ± 0.04 d,e |
| PVOH+BDO | 1.62 ± 0.05 c |
| PVOH+TP | 0.16 ± 0.01 e |
| PVOH+Gly+TP | 2.61 ± 0.08 b |
| PVOH+PDO+TP | 3.70 ± 0.11 a |
| PVOH+BDO+TP | 4.05 ± 0.23 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Szabo, K.; Teleky, B.-E.; Mureșan, V.; Rusu, A.-V.; Socol, C.-T.; Vodnar, D.-C. Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers 2020, 12, 532. https://doi.org/10.3390/polym12030532
Mitrea L, Călinoiu L-F, Martău G-A, Szabo K, Teleky B-E, Mureșan V, Rusu A-V, Socol C-T, Vodnar D-C. Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers. 2020; 12(3):532. https://doi.org/10.3390/polym12030532
Chicago/Turabian StyleMitrea, Laura, Lavinia-Florina Călinoiu, Gheorghe-Adrian Martău, Katalin Szabo, Bernadette-Emoke Teleky, Vlad Mureșan, Alexandru-Vasile Rusu, Claudia-Terezia Socol, and Dan-Cristian Vodnar. 2020. "Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products" Polymers 12, no. 3: 532. https://doi.org/10.3390/polym12030532