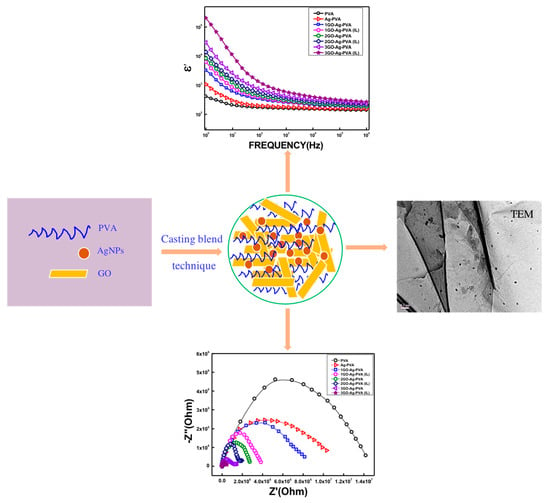
Dielectric Relaxation Behavior of Silver Nanoparticles and Graphene Oxide Embedded Poly(vinyl alcohol) Nanocomposite Film: An Effect of Ionic Liquid and Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Graphene Oxide (GO)
2.3. Nanocomposite Fabrication
2.3.1. Fabrication of PVA and Ag-PVA Film
2.3.2. Fabrication of GO-Ag-PVA and GO-Ag-PVA-IL Composite Film
2.4. Characterization
2.5. Dielectric Relaxation Spectroscopy
3. Results
3.1. Morphological Analysis
3.2. Dielectric Permittivity
3.3. Impedance Analysis
3.4. Nyquist Plot
3.5. AC Conductivity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Verma, S.; Mohanty, S.; Nayaka, S.K. Preparation of hydrophobic epoxy–polydimethylsiloxane–graphene oxide nanocomposite coatings for antifouling application. Soft Matter 2020, 16, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Kurahatti, R.V.; Surendranathan, A.O.; Kori, S.A.; Singh, N.; Ramesh Kumar, A.V.; Srivastava, S. Defence applications of polymer nanocomposites. Def. Sci. J. 2010, 60, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Chakrabarty, D. Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly (vinyl alcohol) and nanocellulose from sugarcane bagasse. J. Ind. Eng. Chem. 2014, 20, 462–473. [Google Scholar] [CrossRef]
- Dhand, V.; Mittal, G.; Rhee, K.Y.; Park, S.J.; Hui, D. A short review on basalt fiber reinforced polymer Composites. Compos. Part B Eng. 2015, 73, 166–180. [Google Scholar] [CrossRef]
- Dhand, V.; Hong, S.K.; Li, L.; Kim, J.M.; Kim, S.H.; Rhee, K.Y.; Lee, W.H. Fabrication of robust, ultrathin and light weight, hydrophilic, PVDF-CNT membrane composite for salt rejection. Compos. Part B Eng. 2019, 160, 632–643. [Google Scholar] [CrossRef]
- Thabet, A.; Ebnalwaled, A.A. Improvement of surface energy properties of PVC nanocomposites for enhancing electrical applications. Measurement 2017, 110, 78–83. [Google Scholar] [CrossRef]
- Subramaniam, K.; Steinhauser, D.; Kluppel, M.; Heinrich, G. Effect of ionic liquid on dielectric, mechanical and dynamic mechanical properties of multiwalled carbon nanotubes/polychloroprene rubber composites. Eur. Polym. J. 2011, 47, 2234–2243. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Lee, J.S.; Jang, J. Polypropylene/Polyaniline Nanofiber/Reduced Graphene Oxide Nanocomposite with enhanced electrical, Dielectric, and Ferroelectric Properties for a High Energy Density Capacitor. ACS Appl. Mater. Interfaces 2015, 7, 22301–22314. [Google Scholar] [CrossRef]
- Shin, E.Y.; Cho, H.J.; Jung, S.; Yang, C.; Noh, Y.Y. A High-k Fluorinated P(VDF-TrFE)-g-PMMA Gate Dielectric for High-Performance Flexible Field-Effect Transistors. Adv. Funct. Mater. 2018, 28, 1704780. [Google Scholar] [CrossRef]
- Martin, M.; Prasad, N.; Sivalingam, M.M.; Sasti kumar, D.; Karthikeyan, B. Optical, phonon properties of ZnO– PVA, ZnO–GO–PVA nanocomposite free standing polymer films for UV sensing. J. Mater. Sci. Mater. Electron. 2018, 29, 365–373. [Google Scholar] [CrossRef]
- Mathen, J.J.; Madhavan, J.; Thomas, A.; Edakkara, A.J.; Sebastian, J.; Joseph, G.P. Transparent ZnO–PVA binary composite for UV- A photo detector: Optical, electrical and thermal properties followed by laser induced fluorescence. J. Mater. Sci. Mater. Electron. 2017, 28, 7190–7203. [Google Scholar] [CrossRef]
- Wang, Z.; Han, N.M.; Wu, Y.; Liu, X.; Shen, X.; Zheng, Q.; Kim, J.K. Ultrahigh dielectric constant and low loss of highly-aligned graphene aerogel/poly(vinyl alcohol) composites with insulating barriers. Carbon 2017, 123, 385–394. [Google Scholar] [CrossRef]
- Pawar, S.P.; Biswas, S.; Kar, G.P.; Bose, S. High frequency millimetre wave absorbers derived from polymeric nanocomposites. Polymer 2016, 84, 398–419. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, T.Y.; Suk, J.W.; Chou, H.; Jang, J.H.; Lee, J.H.; Kholmanov, I.N.; Akinwande, D.; Ruoff, R.S. Enhanced Dielectric Performance in Polymer Composite Films with Carbon Nanotube-Reduced Graphene Oxide Hybrid Filler. Small 2014, 10, 3405–3411. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, highly reduced graphene oxide and graphene: Versatile building blocks for carbon based –materials. Small 2010, 6, 711. [Google Scholar] [CrossRef]
- Sheshmani, S.; Ashori, A.; Fashapoyeh, M.A. Wood plastic composite using graphene nanoplatelets. Int. J. Biol. Macromol. 2013, 58, 1. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Kim, H.J.; Jung, D.H. A Comprehensive Review of Graphene Nanocomposites: Research Status and Trends. J. Nanomater. 2013, 2013, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterization of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular Level Dispersion of Graphene into Poly(vinyl alcohol ) and Effective reinforcement of Their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property of relationships of polycarbonate and graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Sadasivuni, K.K.; Ponnamma, D.; Almadeed, A.A.; Pasha, S.K.K.; Deshmukh, R.R.; Chidambaram, K. Graphene oxide reinforced poly (4-styrenesulfonic acid)/polyvinyl alcohol blend composites with enhanced dielectric properties for portable and flexible electronics. Mater. Chem. Phys. 2017, 186, 188–201. [Google Scholar] [CrossRef]
- Abargues, R.; Marqués-Hueso, J.; Canet-Ferrer, J.; Pedrueza, E.; Valdés, J.L.; Jiménez, E.; Martínez-Pastor, J.P. High-resolution electron-beam patternable nanocomposite containing metal nanoparticles for plasmonics. Nanotechnol. 2008, 19, 355308–355314. [Google Scholar] [CrossRef] [PubMed]
- Abargues, R.; Gradess, R.; Canet-Ferrer, J.; Abderrafi, K.; Valdes, J.L.; Martinez-Pastor, J. Scalable heterogeneous synthesis of metallic nanoparticles and aggregates with polyvinyl alcohol. J New Chem. 2009, 33, 913–917. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Development and characterization of nano-crystalline cellulose incorporated Poly(lactic acid) composite films. J. Mater. Sci. Mater. Electron. 2017, 28, 13401. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Investigation of structural and thermal properties of distinct nanofillers doped PVA composite films. Polym. Bull. 2018, 76, 73–86. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Fabrication of reduced graphene oxide nanosheets doped PVA composite films for tailoring their Opto-mechanical properties. Appl. Phys. 2017, 123, 424. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Investigation of Zinc Oxide –Loaded Poly(vinyl alcohol) nanocomposite films in tailoring their structural, optical and mechanical Properties. J. Electron. Mater. 2018, 47, 3912. [Google Scholar] [CrossRef]
- Rao, J.K.; Raizada, A.; Ganguly, D.; Mankad, M.M.; Satayanarayana, S.V.; Madhu, G.M.J. Mater. Sci. 2015, 50, 7064.
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. 2016, 58, 36–43. [Google Scholar] [CrossRef]
- Dawy, M.; Rifaat, H.M.; Menazea, A.A. Characterization of Ag nanoparticles by nanosecond pulsed laser ablation doped in chitosan. Curr. Sci. Int. 2015, 4, 621–627. [Google Scholar]
- Menazea, A.A.; Elashmawi, I.S.; Abd El-kader, F.H.; Hakeem, N.A. Nanosecond pulsed laser ablation in liquids as new route for preparing polyvinyl carbazole/silver nanoparticles composite: Spectroscopic and thermal studies. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2564–2571. [Google Scholar] [CrossRef]
- Twu, Y.K.; Chen, Y.W.; Shih, C.M. Preparation of silver nanoparticles using chitosan suspensions. Powder Technol. 2008, 185, 251–257. [Google Scholar] [CrossRef]
- Ananth, A.N.; Umapathy, S. On the optical and thermal properties of in situ/ex situ reduced Ag NP’s/PVA composites and its role as a simple SPR-based protein sensor. Appl. Nanosci. 2011, 1, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Mahendia, S.; Tomar, A.K.; Kumar, S. Electrical conductivity and dielectric spectroscopic studies of PVA–Ag nanocomposite films. J. Alloys Compd. 2010, 508, 406–411. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J.X. Langmyir–Blodgett assembly of graphite oxide single layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef]
- Ramasanta, L.J.; Hernandez, M.; Lopez-Manchaob, M.A.; Verdejo, R. Large-Surface-area BN nanosheets and their utilization in polymeric composites with improved thermal and dielectric properties. Nanoscale Res. Lett. 2011, 6, 508–513. [Google Scholar]
- Brochu, P.; Pei, Q. Advances in Dielectric Elastomers for Actuators and Artificial Muscles Macromol. Rapid Commun. 2010, 31, 10–36. [Google Scholar] [CrossRef]
- Li, R.; Xiong, C.; Kuang, D.; Dong, L.; Lei, Y.; Yao, J.; Jiang, M.; Li, L. Polyamide 11/Poly(vinylidene fluoride) Blends as Novel Flexible Materials for Capacitors. Macromol. Rapid Commun. 2008, 29, 1449–1454. [Google Scholar] [CrossRef]
- Singh, V.P.; Ramani, R.; Singh, A.S.; Mishra, P.; Pal, V.; Saraiya, A. Dielectric and conducting behavior of pyrene functionalized PANI/P(VDF-co-HFP) blend. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Fan, B.H.; Zha, J.W.; Wang, D.; Zhao, J.; Dang, Z.M. Experimental study and theoretical prediction of dielectric permittivity in BaTiO3/polyimide nanocomposite films. Appl. Phys. Lett. 2012, 100, 092903. [Google Scholar] [CrossRef]
- Ku, C.C.; Liepins, R. Chemical principles. In Electrical Properties of Polymers; Hanser Publishers: Munich, New York, USA, 1987. [Google Scholar]
- Ying, X.; Yuezhen, B.; Chiang, C.K.; Masaru, M. Dielectric effects on positive temperature coefficient composites of polyethylene and short carbon fibers. Carbon 2007, 45, 1302. [Google Scholar]
- Sahoo, B.P.; Naskar, K.; Tripathy, D.K. Conductive carbon black-filled ethylene acrylic elastomer vulcanizates: Physico-mechanical, thermal, and electrical properties. J. Mater. Sci. 2012, 47, 2421–2433. [Google Scholar] [CrossRef]
- Ferloni, P.; Mastragostino, M.; Meneghello, L. Impedance analysis of electronically conducting polymers. Electrochim. Acta 1996, 41, 27–33. [Google Scholar] [CrossRef]
- Mohanraj, G.T.; Chaki, T.K.; Chakraborty, A.; Khastgir, D. AC impedance analysis and EMI shielding effectiveness of conductive SBR composites. Polym. Eng. Sci. 2006, 46, 1342. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Raja, V.; Sharma, A.K.; Rao, V.V.R.N. Impedance spectroscopic and dielectric analysis of PMMA-CO-P4VPNO polymer film. Mater. Lett. 2004, 58, 3242–3247. [Google Scholar] [CrossRef]
- Sahoo, B.P.; Naskar, K.; Dubey, K.A.; Choudhary, R.N.P.; Tripathy, D.K. Study of dielectric relaxation behavior of electron beam-cured conductive carbon black-filled ethylene acrylic elastomer. J. Mater. Sci. 2013, 48, 702–713. [Google Scholar] [CrossRef]
- Li, J.; Kim, J.-K. Percolation threshold of conducting polymer composites containing 3D randomly distributed graphite nanoplatelets. Compos. Sci. Technol. 2007, 67, 2114. [Google Scholar] [CrossRef] [Green Version]
- Jager, K.M.; McQueen, D.H.; Tchmutin, I.A.; Ryvkina, N.G.; Kluppel, M. Electron transport and ac electrical properties of carbon black polymer composite. J. Phys. D 2001, 34, 2699. [Google Scholar] [CrossRef]
- Nath, A.K.; Kumar, A. Ionic liquid based polymer electrolyte dispersed with doped polyaniline nanorods. Solid State Ion. 2013, 253, 8–17. [Google Scholar] [CrossRef]
- Zhang, W.; Dehghani-Sanij, A.A.; Blackburn, R.S. Carbon based conductive polymer composites. J. Mater. Sci. 2007, 42, 3408. [Google Scholar] [CrossRef]
- Jing, X.; Zhao, W.; Lan, L. The effect of particle size on electronic conducting percolation threshold in polymer/conducting particle composites. J. Mater. Sci. Lett. 2000, 19, 377. [Google Scholar] [CrossRef]
- Halder, N.C. Tunneling and Hopping Parameters in Ta-Oxide Thick Films. Electrocomp. Sci. Technol. 1983, 11, 21. [Google Scholar] [CrossRef] [Green Version]











| Sample Codes | σdc (S/cm) | A | s |
|---|---|---|---|
| PVA | 7.65 × 10−6 | 1.70 × 10−6 | 4.73 × 10−5 |
| Ag-PVA | 1.54 × 10−5 | 1.37 × 10−4 | 4.98 × 10−7 |
| 1GO-Ag-PVA | 2.17 × 10−5 | 1.44 × 10−4 | 5.89 × 10−7 |
| 1GO-Ag-PVA(IL) | 2.22 × 10−5 | 8.37 × 10−5 | 1.48 × 10−6 |
| 2GO-Ag-PVA | 2.52 × 10−5 | 2.81 × 10−6 | 3.31 × 10−5 |
| 2GO-Ag-PVA(IL) | 3.41 × 10−5 | 4.17 × 10−5 | 3.49 × 10−6 |
| 3GO-Ag-PVA | 5.68 × 10−5 | 1.17 × 10−6 | 1.34 × 10−4 |
| 3GO-Ag-PVA(IL) | 1.46 × 10−4 | 1.28 × 10−4 | 1.55 × 10−6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, G.; Das, M.; Yadav, M.; Sahoo, B.P.; Tripathy, J. Dielectric Relaxation Behavior of Silver Nanoparticles and Graphene Oxide Embedded Poly(vinyl alcohol) Nanocomposite Film: An Effect of Ionic Liquid and Temperature. Polymers 2020, 12, 374. https://doi.org/10.3390/polym12020374
Sahu G, Das M, Yadav M, Sahoo BP, Tripathy J. Dielectric Relaxation Behavior of Silver Nanoparticles and Graphene Oxide Embedded Poly(vinyl alcohol) Nanocomposite Film: An Effect of Ionic Liquid and Temperature. Polymers. 2020; 12(2):374. https://doi.org/10.3390/polym12020374
Chicago/Turabian StyleSahu, Ganeswar, Mamata Das, Mithilesh Yadav, Bibhu Prasad Sahoo, and Jasaswini Tripathy. 2020. "Dielectric Relaxation Behavior of Silver Nanoparticles and Graphene Oxide Embedded Poly(vinyl alcohol) Nanocomposite Film: An Effect of Ionic Liquid and Temperature" Polymers 12, no. 2: 374. https://doi.org/10.3390/polym12020374