Integrating Benzenesulfonic Acid Pretreatment and Bio-Based Lignin-Shielding Agent for Robust Enzymatic Conversion of Cellulose in Bamboo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatments
2.3. Solid Substrates Composition and Pretreatment Liquor Analysis
2.4. Lignin Separation from BA-W Pretreatment Hydrolysate
2.5. Enzymatic Hydrolysis
2.6. Characterization of Raw Material and Solid Substrates
2.7. DLS Analysis
3. Results and Discussion
3.1. Effects of Different Pretreatments on the components Removal and Cellulose Enzymatic Conversion
3.2. Preliminary Optimization of BA-W Pretreatment
3.3. Components Removal and Enzymatic Conversion of Substrates Pretreated at Optimized Conditions
3.4. Characterizations of the Substrates Pretreated at Optimized Conditions
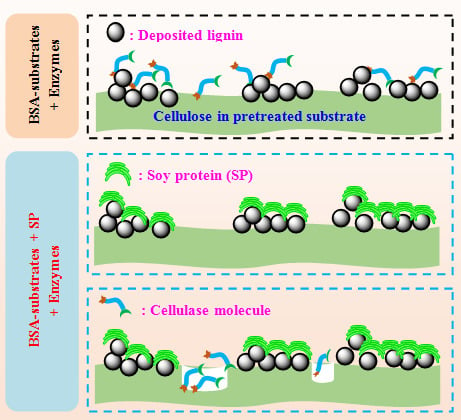
3.5. Improving the Enzymatic Conversion of Cellulose in Pretreated Substrates by Inexpensive Soy Protein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isikgor, F.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, F.; Jungmeier, G.; Wellisch, M.; Willke, T.; Skiadas, I.; Van Ree, R.; de Jong, E. Toward a common classification approach for biorefinery systems. Biofuels Bioprod. Bioref. 2010, 3, 534–546. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.Y.; Pan, X.; Zalesny, R.S. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies, and recalcitrance. Appl. Microbiol. Biotechnol. 2010, 87, 847–857. [Google Scholar] [CrossRef]
- Zhao, X.B.; Cheng, K.; Liu, D.H. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Zhao, X.B.; Li, S.M.; Wu, R.C.; Liu, D.H. Organosolv fractionating pretreatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Bioref. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Sun, Y.; Hu, L.; Liu, S.; Lei, T.; et al. Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. ChemSusChem 2017, 10, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dou, J.; Ma, Q.; Li, N.; Wu, R.; Yelle, D.J.; Vuorinen, T.; Fu, S.; Pan, X.; Zhu, J.Y. Rapid and near-complete dissolution of wood lignin at ≤80 °C by a recyclable acid hydrotrope. Sci. Adv. 2017, 3, e1701735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgdon, T.; Kaler, E. Hydrotropic solutions. Curr. Opin. Colloid Interface Sci. 2007, 12, 121–128. [Google Scholar] [CrossRef]
- Kunz, W.; Holmberg, K.; Zemb, T. Hydrotropes. Curr. Opin. Colloid Interface Sci. 2016, 22, 99–107. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef]
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of surfactant in enzymatic hydrolysis of lignocellulose. Enzyme Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnol. Bioeng. 2006, 94, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Liu, J.; Zheng, P.; Li, M.; Zhou, Y.; Huang, L.; Chen, L.; Shuai, L. Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnol. Biofuels 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio, C.; Badino, A.C.; Farinas, C.S. Soybean protein as a cost-effective lignin-blocking additive for the saccharification of sugarcane bagasse. Bioresour. Technol. 2016, 221, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Qiu, X.; Zhu, D.; Li, Z.; Zhan, N.; Zheng, J.; Lou, H.; Zhou, M.; Yang, D. Effect of the molecular structure of lignin-based polyoxyethylene ether on enzymatic hydrolysis efficiency and kinetics of lignocelluloses. Bioresour. Technol. 2015, 193, 266–273. [Google Scholar] [CrossRef]
- Cai, C.; Qiu, X.; Zeng, M.; Lin, M.; Lin, X.; Lou, H.; Zhan, X.; Pang, Y.; Huang, J.; Xie, L. Using polyvinylpyrrolidone to enhance the enzymatic hydrolysis of lignocelluloses by reducing the cellulase non-productive adsorption on lignin. Bioresour. Technol. 2016, 227, 74–78. [Google Scholar] [CrossRef]
- Akimkulova, A.; Zhou, Y.; Zhao, X.; Liu, D. Improving the enzymatic hydrolysis of dilute acid pretreated wheat straw by metal ion blocking of non-productive cellulase adsorption on lignin. Bioresour. Technol. 2016, 208, 110–116. [Google Scholar] [CrossRef]
- Lee, W.G.; Lee, J.S.; Lee, J.P.; Shin, C.S.; Kim, M.S.; Park, S.C. Effect of surfactants on ethanol fermentation using glucose and cellulosic hydrolyzates. Biotechnol. Lett. 1996, 18, 299–304. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure (LAP); Technical Report (NREL/TP-510-42618); National Renewable Energy Laboratory: Golden, CO, USA, April 2008. [Google Scholar]
- Laine, J.; Stenius, P.; Carlsson, G.; Ström, G. Surface characterization of unbleached kraft pulps by means of ESCA. Cellulose 1994, 1, 145–160. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Gleisner, R.; Dai, H.; Zhu, J.Y. Producing wood-based nanomaterials by rapid fractionation of wood at 80 °C using a recyclable acid hydrotrop. Green Chem. 2017, 19, 3370–3379. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B. A comparison of dilute aqueous p-toluenesulfonic and sulfuric acid pretreatments and saccharification of corn stover at moderate temperatures and pressures. Bioresour. Technol. 2012, 125, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Grattoni, C.A.; Dawe, R.A.; Seah, C.Y.; Gray, J.D. Lower critical solution coexistence curve and physical properties (density, viscosity, surface tension and interfacial tension) of 2, 6-lutidine plus water. J. Chem. Eng. Data 1993, 38, 516–519. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.; Gong, Z.; Yang, G.; Li, R.; Chen, L.; Huang, L.; Luo, X.L. Near-complete removal of non-cellulosic components from bamboo by 1-pentanol induced organosolv pretreatment under mild conditions for robust cellulose enzymatic hydrolysis. Cellulose 2019, 26, 3801–3814. [Google Scholar] [CrossRef]
- Leschinsky, M.; Zuckerstätter, G.; Weber, H.K.; Patt, R.; Sixta, H. Effect of autohydrolysis of Eucalyptus globulus wood on lignin structure. Part 2: Influence of autohydrolysis intensity. Holzforschung 2008, 62, 653–658. [Google Scholar] [CrossRef]
- Luo, X.L.; Liu, J.; Wang, H.S.; Huang, L.L.; Chen, L.H. Comparison of hot-water extraction and steam treatment for production of high purity-grade dissolving pulp from green bamboo. Cellulose 2014, 21, 1445–1457. [Google Scholar] [CrossRef] [Green Version]
- Donohoe, B.S.; Decker, S.R.; Tucker, M.P.; Himmel, M.E.; Vinzant, T.B. Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 2008, 101, 913–925. [Google Scholar] [CrossRef]
- Gao, D.; Haarmeyer, C.; Balan, V.; Whitehead, T.A.; Dale, B.E.; Chundawat, S.P. Lignin triggers irreversible cellulase loss during pretreated lignocellulosic biomass saccharification. Biotechnol. Biofuels 2014, 7, 175. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wyman, C.E. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004, 86, 88–98. [Google Scholar] [CrossRef]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Grethlein, H.E. The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulose substrates. Nat. Biotechnol. 1985, 3, 155–160. [Google Scholar] [CrossRef]
- Xiang, L.; Yi, Z. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar]
- Liu, J.; Li, R.Q.; Shuai, L.; You, J.H.; Zhao, Y.B.; Chen, L.; Li, M.; Chen, L.H.; Huang, L.L.; Luo, X.L. Comparison of liquid hot water (LHW) and high boiling alcohol/water (HBAW) pretreatments for improving enzymatic saccharification of cellulose in bamboo. Ind. Crop. Prod. 2017, 107, 139–148. [Google Scholar] [CrossRef]
- Qin, C.; Clarke, K.; Li, K. Interactive forces between lignin and cellulase as determined by atomic force microscopy. Biotechnol. Biofuels 2014, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Sun, S.; Huang, C.; Yong, Q.; Elder, T.; Tu, M. Stimulation and inhibition of enzymatic hydrolysis by organosolv lignins as determined by zeta potential and hydrophobicity. Biotechnol. Biofuels 2017, 10, 162. [Google Scholar] [CrossRef] [Green Version]
- Karimi, K.; Taherzadeh, M.J. A critical review on analysis in pretreatment of lignocelluloses: Degree of polymerization, adsorption/desorption, and accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2015, 108, 22–30. [Google Scholar] [CrossRef]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Formic acid based organosolv pulping of bamboo (Phyllostachys acuta): Comparative characterization of the dissolved lignins with milled wood lignin. Chem. Eng. J. 2012, 179, 80–89. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Gong, Z.; Shi, J.; Chen, L.; Zhu, W.; Zhou, Y.; Huang, L.; Liu, J. Integrating Benzenesulfonic Acid Pretreatment and Bio-Based Lignin-Shielding Agent for Robust Enzymatic Conversion of Cellulose in Bamboo. Polymers 2020, 12, 191. https://doi.org/10.3390/polym12010191
Luo X, Gong Z, Shi J, Chen L, Zhu W, Zhou Y, Huang L, Liu J. Integrating Benzenesulfonic Acid Pretreatment and Bio-Based Lignin-Shielding Agent for Robust Enzymatic Conversion of Cellulose in Bamboo. Polymers. 2020; 12(1):191. https://doi.org/10.3390/polym12010191
Chicago/Turabian StyleLuo, Xiaolin, Zhenggang Gong, Jinghao Shi, Lihui Chen, Wenyuan Zhu, Yonghui Zhou, Liulian Huang, and Jing Liu. 2020. "Integrating Benzenesulfonic Acid Pretreatment and Bio-Based Lignin-Shielding Agent for Robust Enzymatic Conversion of Cellulose in Bamboo" Polymers 12, no. 1: 191. https://doi.org/10.3390/polym12010191