A Novel Electroactive Imide Oligomer and Its Application in Anticorrosion Coating
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instrumentation
2.2. Synthesis of Aniline Tetramer (AT)
2.3. Synthesis of Polyimide (PI)
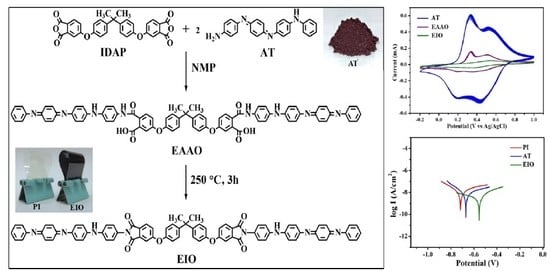
2.4. Synthesis of Electroactive Amic Acid Oligomer (EAAO) and Imide Oligomer (EIO)
2.5. Reduction of AT and EAAO
2.6. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of AT
3.2. Characterization of EAAO and EIO
3.3. Chemical Oxidation of AT and EAAO
3.4. Electroactivity of AT, EAAO and EIO Coatings
3.5. Potentiodynamic Measurements
3.6. Electrochemical Impedance Spectroscopy (EIS) Measurements
3.7. Corrosion Products and Corrosion Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- George, F.; Hays, P. Now is the Time; World Corrosion Organization: New York, NY, USA, 2016. [Google Scholar]
- Li, P.; He, X.; Huang, T.-C.; White, K.L.; Zhang, X.; Liang, H.; Nishimura, R.; Sue, H.-J. Highly effective anti-corrosion epoxy spray coatings containing self-assembled clay in smectic order. J. Mater. Chem. A 2015, 3, 2669–2676. [Google Scholar] [CrossRef]
- Li, P.; Huang, T.-C.; White, K.L.; Hawkins, S.; Kotaki, M.; Nishimura, R.; Sue, H.-J. Spray-coated epoxy barrier films containing high aspect ratio functionalized graphene nanosheets. RSC Adv. 2015, 5, 102633–102642. [Google Scholar] [CrossRef]
- Huang, T.-C.; Lai, G.-H.; Li, C.-E.; Tsai, M.-H.; Wan, P.-Y.; Chung, Y.-H.; Lin, M.-H. Advanced anti-corrosion coatings prepared from [small alpha]-zirconium phosphate/polyurethane nanocomposites. RSC Adv. 2017, 7, 9908–9913. [Google Scholar] [CrossRef] [Green Version]
- Lai, G.H.; Huang, T.C.; Tseng, I.H.; Huang, B.S.; Yang, T.I.; Tsai, M.H. Transparency anticorrosion coatings prepared from alumina-covered graphene oxide/polyimide nanocomposites. Express Polym. Lett. 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Comparison of electrochemical corrosion behaviour of mgo and zro2 coatings on am50 magnesium alloy formed by plasma electrolytic oxidation. Corros. Sci. 2009, 51, 2483–2492. [Google Scholar] [CrossRef] [Green Version]
- Palanivel, V.; Zhu, D.; van Ooij, W.J. Nanoparticle-filled silane films as chromate replacements for aluminum alloys. Prog. Org. Coat. 2003, 47, 384–392. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.-G.; Zhuang, J.-J.; Song, R.-X.; Lu, X.-Y.; Su, X.-P. Effects of current density on microstructure and properties of plasma electrolytic oxidation ceramic coatings formed on 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 806–813. [Google Scholar] [CrossRef]
- Anitha, R.; Chitra, S.; Hemapriya, V.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Implications of eco-addition inhibitor to mitigate corrosion in reinforced steel embedded in concrete. Constr. Build. Mater. 2019, 213, 246–256. [Google Scholar] [CrossRef]
- Ituen, E.B.; Akaranta, O.; Umoren, S.A. N-acetyl cysteine based corrosion inhibitor formulations for steel protection in 15% hcl solution. J. Mol. Liq. 2017, 246, 112–118. [Google Scholar] [CrossRef]
- Peng, S.; Zhao, W.; Li, H.; Zeng, Z.; Xue, Q.; Wu, X. The enhancement of benzotriazole on epoxy functionalized silica sol–gel coating for copper protection. Appl. Surf. Sci. 2013, 276, 284–290. [Google Scholar] [CrossRef]
- Eduok, U.; Suleiman, R.; Gittens, J.; Khaled, M.; Smith, T.J.; Akid, R.; El Ali, B.; Khalil, A. Anticorrosion/antifouling properties of bacterial spore-loaded sol–gel type coating for mild steel in saline marine condition: A case of thermophilic strain of bacillus licheniformis. RSC Adv. 2015, 5, 93818–93830. [Google Scholar] [CrossRef] [Green Version]
- Rohwerder, M.; Michalik, A. Conducting polymers for corrosion protection: What makes the difference between failure and success? Electrochim. Acta 2007, 53, 1300–1313. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Jen, C.-C.; Huang, H.-Y.; Wu, P.-C.; Huang, C.-C.; Yeh, J.-M. Preparation and properties of heterocyclically conjugated poly(3-hexylthiophene)–clay nanocomposite materials. J. Appl. Polym. Sci. 2004, 91, 3438–3446. [Google Scholar] [CrossRef]
- Hosseini, M.; Fotouhi, L.; Ehsani, A.; Naseri, M. Enhancement of corrosion resistance of polypyrrole using metal oxide nanoparticles: Potentiodynamic and electrochemical impedance spectroscopy study. J. Colloid Interface Sci. 2017, 505, 213–219. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline composites with metals, metalloids and nonmetals. Synth. Met. 2013, 170, 31–56. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Cui, M.; Li, W.; Zhao, H.; Wang, L. Corrosion protection performance of waterborne epoxy coatings containing self-doped polyaniline nanofiber. Appl. Surf. Sci. 2017, 407, 213–222. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Das, R.; Prusty, G.; Sahu, D.; Swain, S.K. Anticorrosion performance of three-dimensional hierarchical pani@bn nanohybrids. Ind. Eng. Chem. Res. 2016, 55, 2921–2931. [Google Scholar] [CrossRef]
- Grgur, B.N. On the role of aniline oligomers on the corrosion protection of mild steel. Synth. Met. 2014, 187, 57–60. [Google Scholar] [CrossRef]
- Gu, L.; Liu, S.; Zhao, H.; Yu, H. Anticorrosive oligoaniline-containing electroactive siliceous hybrid materials. RSC Adv. 2015, 5, 56011–56019. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lee, Y.-T.; Yeh, L.-C.; Jian, J.-W.; Huang, T.-C.; Liang, H.-T.; Yeh, J.-M.; Chou, Y.-C. Photoactively electroactive polyamide with azo group in the main chain via oxidative coupling polymerization. Polym. Chem. 2013, 4, 343–350. [Google Scholar] [CrossRef]
- Yeh, L.-C.; Huang, T.-C.; Huang, Y.-P.; Huang, H.-Y.; Chen, H.-H.; Yang, T.-I.; Yeh, J.-M. Synthesis electroactive polyurea with aniline-pentamer-based in the main chain and its application in electrochemical sensor. Electrochim. Acta 2013, 94, 300–306. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Tran, H.D.; Mecklenburg, M.; Guan, X.N.; Stieg, A.Z.; Regan, B.C.; Martin, D.C.; Kaner, R.B. Morphological and dimensional control via hierarchical assembly of doped oligoaniline single crystals. J. Am. Chem. Soc. 2012, 134, 9251–9262. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yu, Z.; Hu, J.; Chandrasekaran, S.; Lindsay, D.M.; Wei, Z.; Faul, C.F.J. Block-like electroactive oligo(aniline)s: Anisotropic structures with anisotropic function. J. Mater. Chem. 2012, 22, 16230–16234. [Google Scholar] [CrossRef]
- Sun, N.; Meng, S.; Chao, D.; Zhou, Z.; Du, Y.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. Highly stable electrochromic and electrofluorescent dual-switching polyamide containing bis(diphenylamino)-fluorene moieties. Polym. Chem. 2016, 7, 6055–6063. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, N.; Li, F.; Jia, X.; Wang, C.; Chao, D. Multiple stimuli-responsive fluorescence behavior of novel polyamic acid bearing oligoaniline, triphenylamine, and fluorene groups. ACS Appl. Mater. Interfaces 2017, 9, 6497–6503. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, X.; Feng, M.; Wang, C.; Chao, D. Synthesis and electrochemical characterization of polyamic acid containing oligoaniline and triphenylamine. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1669–1673. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-Y.; Huang, T.-C.; Lin, J.-C.; Chang, J.-H.; Lee, Y.-T.; Yeh, J.-M. Advanced environmentally friendly coatings prepared from amine-capped aniline trimer-based waterborne electroactive polyurethane. Mater. Chem. Phys. 2013, 137, 772–780. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Li, X.; Qiu, S.; Ye, Y.; Yang, F.; Zhao, H. Effect of self-assembled tetraaniline nanofiber on the anticorrosion performance of waterborne epoxy coating. Prog. Org. Coat. 2019, 128, 137–147. [Google Scholar] [CrossRef]
- Huang, C.-C.; Jang, G.-W.; Chang, K.-C.; Hung, W.-I.; Yeh, J.-M. High-performance polyimide–clay nanocomposite materials based on a dual intercalating agent system. Polym. Int. 2008, 57, 605–611. [Google Scholar] [CrossRef]
- Huang, T.-C.; Lin, S.-T.; Yeh, L.-C.; Chen, C.-A.; Huang, H.-Y.; Nian, Z.-Y.; Chen, H.-H.; Yeh, J.-M. Aniline pentamer-based electroactive polyimide prepared from oxidation coupling polymerization for electrochemical sensing application. Polymer 2012, 53, 4373–4379. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, L.-C.; Huang, H.-Y.; Nian, Z.-Y.; Yeh, Y.-C.; Chou, Y.-C.; Yeh, J.-M.; Tsai, M.-H. The use of a carbon paste electrode mixed with multiwalled carbon nanotube/electroactive polyimide composites as an electrode for sensing ascorbic acid. Polym. Chem. 2014, 5, 630–637. [Google Scholar] [CrossRef]
- Huang, T.-C.; Hsieh, C.-F.; Yeh, T.-C.; Lai, C.-L.; Tsai, M.-H.; Yeh, J.-M. Comparative studies on corrosion protection properties of polyimide-silica and polyimide-clay composite materials. J. Appl. Polym. Sci. 2011, 119, 548–557. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Huang, T.-C.; Yeh, T.-C.; Tsai, C.-Y.; Lai, C.-L.; Tsai, M.-H.; Yeh, J.-M.; Chou, Y.-C. Advanced anticorrosive materials prepared from amine-capped aniline trimer-based electroactive polyimide-clay nanocomposite materials with synergistic effects of redox catalytic capability and gas barrier properties. Polymer 2011, 52, 2391–2400. [Google Scholar] [CrossRef]
- Lyu, W.; Feng, J.; Yan, W.; Faul, C.F.J. Self-assembly of tetra(aniline) nanowires in acidic aqueous media with ultrasonic irradiation. J. Mater. Chem. C 2015, 3, 11945–11952. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Duran, B.; Turhan, M.C.; Bereket, G.; Saraç, A.S. Electropolymerization, characterization and corrosion performance of poly(n-ethylaniline) on copper. Electrochim. Acta 2009, 55, 104–112. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, T.-C.; Huang, H.-Y.; Ji, W.-F.; Lin, T.-C.; Chen, C.-A.; Yang, T.-I.; Yeh, J.-M. Electrochemical investigations of the anticorrosive and electrochromic properties of electroactive polyamide. Electrochim. Acta 2012, 63, 185–191. [Google Scholar] [CrossRef]
- Sheng, X.; Cai, W.; Zhong, L.; Xie, D.; Zhang, X. Synthesis of functionalized graphene/polyaniline nanocomposites with effective synergistic reinforcement on anticorrosion. Ind. Eng. Chem. Res. 2016, 55, 8576–8585. [Google Scholar] [CrossRef]
- Yeh, L.-C.; Huang, T.-C.; Lin, Y.-J.; Lai, G.-H.; Yang, T.-I.; Lo, A.-Y.; Yeh, J.-M. Electroactive polyamide modified carbon paste electrode for the determination of ascorbic acid. Int. J. Green Energy 2016, 13, 1334–1341. [Google Scholar] [CrossRef]
- Huang, T.C.; Yeh, L.C.; Lai, G.H.; Lai, F.Y.; Yang, T.I.; Huang, Y.J.; Lo, A.Y.; Yeh, J.M. Electroactive polyurea/cnt composite-based electrode for detection of vitamin c. Express Polym. Lett. 2016, 10, 450–461. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, L.-C.; Lai, G.-H.; Huang, B.-S.; Yang, T.-I.; Hsu, S.-C.; Lo, A.-Y.; Yeh, J.-M. Advanced superhydrophobic electroactive fluorinated polyimide and its application in anticorrosion coating. Int. J. Green Energy 2017, 14, 113–120. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, T.-C.; Huang, H.-Y.; Ji, W.-F.; Chou, Y.-C.; Hung, W.-I.; Yeh, J.-M.; Tsai, M.-H. Electrochemical studies on aniline-pentamer-based electroactive polyimide coating: Corrosion protection and electrochromic properties. Electrochim. Acta 2011, 56, 10151–10158. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Wang, M.; Yang, Z.; Pan, Y.; Liu, G. Inhibiting the corrosion-promotion activity of graphene. Chem. Mater. 2015, 27, 2367–2373. [Google Scholar] [CrossRef]
- Wei, H.; Wu, X.; Hu, B.; Fang, X. Preparation, characterization, and properties of poly(thioether ether ketone imide)s from isomeric bis(chlorophthalimide)s. Polym. Adv. Technol. 2011, 22, 2488–2495. [Google Scholar] [CrossRef]
- Yang, R.; Chao, D.; Liu, H.; Berda, E.B.; Wang, S.; Jia, X.; Wang, C. Synthesis, electrochemical properties and inhibition performance of water-soluble self-doped oligoaniline derivative. Electrochim. Acta 2013, 93, 107–113. [Google Scholar] [CrossRef]
- Chao, D.; Jia, X.; Liu, H.; He, L.; Cui, L.; Wang, C.; Berda, E.B. Novel electroactive poly(arylene ether sulfone) copolymers containing pendant oligoaniline groups: Synthesis and properties. J. Od Polymrt Sci. Part A Polym. Chem. 2011, 49, 1605–1614. [Google Scholar] [CrossRef]
- Jia, X.; Chao, D.; Liu, H.; He, L.; Zheng, T.; Bian, X.; Wang, C. Synthesis and properties of novel electroactive poly(amic acid) and polyimide copolymers bearing pendant oligoaniline groups. Polym. Chem. 2011, 2, 1300–1306. [Google Scholar] [CrossRef]
- Lv, W.; Feng, J.; Yan, W. Electrochemical potential-responsive tetra(aniline) nanocapsules via self-assembly. RSC Adv. 2015, 5, 27862–27866. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wang, D.; Cheng, J.; Pang, X.; Qu, W.; Li, C.; Li, S. Facile fabrication of superhydrophobic surface from fluorinated poss acrylate copolymer via one-step breath figure method and its anti-corrosion property. Polymers 2019, 11, 1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.-S.; Chang, S.-J.; Feng, C.-K.; Lin, W.-L.; Liao, S.-C. Plasma deposition and uv light induced surface grafting polymerization of nipaam on stainless steel for enhancing corrosion resistance and its drug delivery property. Polymers 2018, 10, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano-Vaca, M.G.; Gonzalez-Rodriguez, J.G.; Nicho, M.E.; Casales, M.; Salinas-Bravo, V.M. Corrosion protection of carbon steel by thin films of poly(3-alkyl thiophenes) in 0.5m h2so4. Electrochim. Acta 2008, 53, 3500–3507. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Electrochemical impedance spectroscopy (eis) study on corrosion performance of cralsin coated steels in 3.5wt.% nacl solution. Surf. Coat. Technol. 2009, 204, 784–787. [Google Scholar] [CrossRef]
- Deng, W.; Lin, P.; Li, Q.; Mo, G. Ultrafine-grained copper produced by machining and its unusual electrochemical corrosion resistance in acidic chloride pickling solutions. Corros. Sci. 2013, 74, 44–49. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, C.; Lu, H.; Gao, F.; Ma, H. Influence of phytic acid on the corrosion behavior of iron under acidic and neutral conditions. Electrochim. Acta 2014, 150, 188–196. [Google Scholar] [CrossRef]
- Iroh, J.O.; Su, W. Corrosion performance of polypyrrole coating applied to low carbon steel by an electrochemical process. Electrochim. Acta 2000, 46, 15–24. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthukrishnan, S.; Venkatachari, G.; Trivedi, D.C. Corrosion protection of steel by polyaniline (pani) pigmented paint coating. Prog. Org. Coat. 2005, 53, 297–301. [Google Scholar] [CrossRef]
- Mrad, M.; Dhouibi, L.; Triki, E. Dependence of the corrosion performance of polyaniline films applied on stainless steel on the nature of electropolymerisation solution. Synth. Met. 2009, 159, 1903–1909. [Google Scholar] [CrossRef]
- Du, P.; Qiu, S.; Liu, C.; Liu, G.; Zhao, H.; Wang, L. In situ polymerization of sulfonated polyaniline in layered double hydroxide host matrix for corrosion protection. New J. Chem. 2018, 42, 4201–4209. [Google Scholar] [CrossRef]
- De Rosa, R.L.; Earl, D.A.; Bierwagen, G.P. Statistical evaluation of eis and enm data collected for monitoring corrosion barrier properties of organic coatings on al-2024-t3. Corros. Sci. 2002, 44, 1607–1620. [Google Scholar] [CrossRef]
- Luo, B.; Xu, A.; Liang, Y.; Huang, Z.; Qiao, Z.; Xia, D.-H. Evaluation on protective performance of organic coatings by analyzing the change rate of phase angle at high frequency. Int. J. Electrochem. Sci. 2012, 7, 8859–8868. [Google Scholar]
- Yan, J.; Zhang, B.; Wang, Z. Monodispersed ultramicroporous semi-cycloaliphatic polyimides for the highly efficient adsorption of co2, h2 and organic vapors. Polym. Chem. 2016, 7, 7295–7303. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of xps spectra of fe2+ and fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ xps analysis of various iron oxide films grown by no2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Sathiyanarayanan, S.; Muthkrishnan, S.; Venkatachari, G. Corrosion protection of steel by polyaniline blended coating. Electrochim. Acta 2006, 51, 6313–6319. [Google Scholar] [CrossRef]
- Li, S.; Zhao, C.; Gou, H.; Li, H.; Li, Y.; Xiang, D. Synthesis and characterization of aniline-dimer-based electroactive benzoxazine and its polymer. RSC Adv. 2017, 7, 55796–55806. [Google Scholar] [CrossRef] [Green Version]













| NMP | DMF | DMAc | DMSO | THF | CHCl3 | CH2Cl2 | Acetone | Ethanol | |
|---|---|---|---|---|---|---|---|---|---|
| EAAO | + + | + + | + + | + + | + + | + | + | + | + |
| Sample | Electrochemical Measurements | ||||||
|---|---|---|---|---|---|---|---|
| Ecorr (V) | Icorr (A cm−2) | RP (Ω cm2) | CR (mm/year) | ba | bc | Thickness (μm) | |
| Bare | −0.89 | 2.52 × 10−6 | 8.69 | 2.90 × 10−2 | 0.060 | 0.309 | - |
| PI | −0.21 | 1.85 × 10−10 | 3.55 × 108 | 2.17 × 10−6 | 0.305 | 0.300 | 22 ± 1 |
| AT | −0.27 | 4.30 × 10−9 | 1.22 × 107 | 5.03 × 10−5 | 0.260 | 0.226 | 21 ± 1 |
| EIO | −0.24 | 2.86 × 10−9 | 2.88 × 107 | 3.35 × 10−5 | 0.421 | 0.344 | 22 ± 1 |
| Sample | Electrochemical Measurements | ||||||
|---|---|---|---|---|---|---|---|
| Ecorr (V) | Icorr (A cm−2) | RP (Ω cm2) | CR (mm/year) | ba | bc | Thickness (μm) | |
| PI | −0.71 | 8.64 × 10−9 | 3.66 × 106 | 1.01 × 10−4 | 0.162 | 0.132 | 22 ± 1 |
| AT | −0.67 | 7.85 × 10−9 | 5.33 × 106 | 9.19 × 10−5 | 0.269 | 0.150 | 21 ± 1 |
| EIO | −0.55 | 3.32 × 10−9 | 1.33 × 107 | 3.89 × 10−5 | 0.138 | 0.385 | 22 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.-S.; Lai, G.-H.; Yang, T.-I.; Tsai, M.-H.; Chou, Y.-C. A Novel Electroactive Imide Oligomer and Its Application in Anticorrosion Coating. Polymers 2020, 12, 91. https://doi.org/10.3390/polym12010091
Huang B-S, Lai G-H, Yang T-I, Tsai M-H, Chou Y-C. A Novel Electroactive Imide Oligomer and Its Application in Anticorrosion Coating. Polymers. 2020; 12(1):91. https://doi.org/10.3390/polym12010091
Chicago/Turabian StyleHuang, Bi-Sheng, Guan-Hui Lai, Ta-I Yang, Mei-Hui Tsai, and Yi-Chen Chou. 2020. "A Novel Electroactive Imide Oligomer and Its Application in Anticorrosion Coating" Polymers 12, no. 1: 91. https://doi.org/10.3390/polym12010091