Modified Epoxy Resin Synthesis from Phosphorus—Containing Polyol and Physical Changes Studies in the Synthesized Products
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Synthesis of Phosphor-Containing Polyol (P-Polyol)
2.3. Synthesis of P-Polyol Modified Epoxy (PPME)
2.4. Preparation of the Epoxy Compositions Including PPME and Its Curing
2.5. Measurements and Analyses
3. Results and Discussion
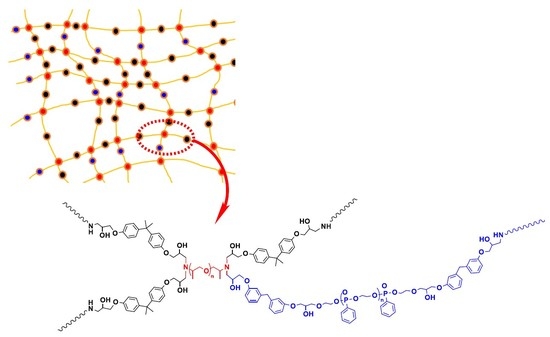
3.1. Structural Characterization of P-Polyol and PPME
3.2. Cure of the Epoxy Compositions and Thermal Properties of Epoxy Polymer
3.3. Mechanical Properties of the Cured Epoxy Compositions
3.4. FE-SEM Images of the Epoxy Polymers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gamardella, F.; Sabatini, V.; Ramis, X.; Serra, A. Tailor-made thermosets obtained by sequential dual-curing combining isocyanate-thiol and epoxy-thiol click reactions. Polymer 2019, 174, 200–209. [Google Scholar] [CrossRef]
- Chu, W.C.; Lin, W.S.; Kuo, S.W. Flexible epoxy resin formed upon blending with a triblock copolymer through reaction-induced microphase separation. Materials 2016, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, L.; Kukla, P.; Eibl, S.; Döring, M. Phosphorus containing polyacrylamides as flame retardants for epoxy-based composites in aviation. Polymers 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Dai, X.; Wang, Y.; Sun, G.; Li, P.; Qu, L.; Sui, Y.; Dou, Y. Preparation and corrosion resistance of ETEO modified graphene oxide/epoxy resin coating. Coatings 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Zhang, Q.; Fang, C.; Chen, J.; Su, J.; Xu, K.; Ai, L.; Liu, D. Preparation, structure, and properties of surface modified graphene/epoxy resin composites for potential application in conductive ink. Coatings 2018, 8, 387. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Tuo, R.; Yang, W.; Zhang, Y.; Xie, Q.; Zha, J.; Lin, J.; He, S. Mechanical, thermal, and electrical properties of bn–epoxy composites modified with carboxyl-terminated butadiene nitrile liquid rubber. Polymers 2019, 11, 1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakopoulos, G.; Masania, K.; Taylor, A.C. Toughening of epoxy using core–shell particles. J. Mater. Sci. 2011, 46, 327–338. [Google Scholar] [CrossRef]
- Liu, S.; Fan, X.; He, C. Improving the fracture toughness of epoxy with nanosilica-rubber core-shell nanoparticles. Compos. Sci. Technol. 2016, 125, 132–140. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.; Lee, D.; Lim, C.S.; Seo, B. Preparation of a branched amine and the physical and thermal studies of the epoxy compositions including the amine compound. J. Appl. Polym. Sci. 2018, 135, 46233. [Google Scholar] [CrossRef]
- Tsang, W.L.; Taylor, A.C. Fracture and toughening mechanisms of silicaand core–shell rubber-toughened epoxy at ambient and low temperature. J. Mater. Sci. 2019, 54, 13938–13958. [Google Scholar] [CrossRef] [Green Version]
- Mariappan, T.; Wilkie, C.A. Flame retardant epoxy resin for electrical and electronic applications. Fire Mater. 2014, 38, 588–598. [Google Scholar] [CrossRef]
- Wang, N.; Teng, H.; Yang, F.; You, J.; Zhang, J.; Wang, D. Synthesis of K-carrageenan flame-retardant microspheres and its application for waterborne epoxy resin with functionalized graphene. Polymers 2019, 11, 1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Teng, H.; Zhang, X.; Zhang, J.; Li, L.; Zhang, J.; Fang, Q. Synthesis of a carrageenan–iron complex and its effect on flame retardancy and smoke suppression for waterborne epoxy. Polymers 2019, 11, 1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifah, A.; Salmeia, S.G. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar]










| Components (g) | Reference | PPME-5 | PPME-10 | PPME-15 |
|---|---|---|---|---|
| DGEBA | 100 (26.7 mol) | 100 | 100 | 100 |
| D-230 | 32.1 (14.0 mol) | 32.1 | 32.1 | 32.1 |
| PPME | 0 | 5 (0.49 mol) | 10 (0.99 mol) | 15 (1.5 mol) |
| Binder | PPME-5 | PPME-10 | PPME-15 | |
|---|---|---|---|---|
| HRR (W/g) | 628.9 | 406.7 | 318.7 | 289.9 |
| Total HR (kJ/g) | 26.1 | 23.4 | 21.1 | 19.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.B.; Kim, T.H.; Kim, T.; Kim, H.J.; Seo, B.; Lim, C.-S.; Lee, W. Modified Epoxy Resin Synthesis from Phosphorus—Containing Polyol and Physical Changes Studies in the Synthesized Products. Polymers 2019, 11, 2116. https://doi.org/10.3390/polym11122116
Jang JB, Kim TH, Kim T, Kim HJ, Seo B, Lim C-S, Lee W. Modified Epoxy Resin Synthesis from Phosphorus—Containing Polyol and Physical Changes Studies in the Synthesized Products. Polymers. 2019; 11(12):2116. https://doi.org/10.3390/polym11122116
Chicago/Turabian StyleJang, Jeong Beom, Tae Hee Kim, Taeyoon Kim, Hye Jin Kim, Bongkuk Seo, Choong-Sun Lim, and Wonjoo Lee. 2019. "Modified Epoxy Resin Synthesis from Phosphorus—Containing Polyol and Physical Changes Studies in the Synthesized Products" Polymers 11, no. 12: 2116. https://doi.org/10.3390/polym11122116