Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dual Crosslinked Collagen–Chitosan Film
2.3. Characterization of Films
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Scanning Electron Microscopy
2.3.3. Thermogravimetric Analysis
2.3.4. Swelling and Invitro Degradation Studies
2.3.5. Cell Adhesion and Proliferation Studies
3. Results and Discussion
3.1. FTIR Analysis of Films
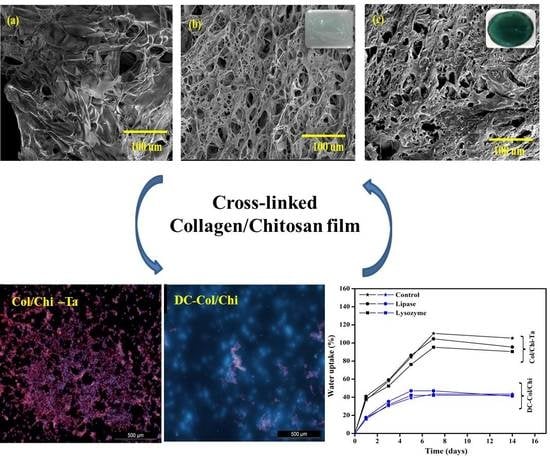
3.2. SEM Micrographs
3.3. TGA Analysis
3.4. Swelling and In Vitro Degradation Studies
3.5. Cell Adhesion and Proliferation Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fiejdasz, S.; Szczubiałka, K.; Lewandowska-Lancucka, J.; Osyczka, A.M.; Nowakowska, M. Biopolymer-based hydrogels as injectable materials for tissue repair scaffolds. Biomed. Mater. 2013, 8, 035013. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Ku, H.F.; Rajesh, R. Chitosan/γ-poly(glutamic acid) scaffolds with surface-modified albumin, elastin and poly-L-lysine for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. C 2017, 78, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lactic-coglycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Yamane, S.; Iwasaki, N.; Majima, T.; Funakoshi, T.; Masuko, T.; Harad, K.; Minami, A.; Monde, K.; Nishimura, S.-h. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials 2005, 26, 611–619. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z.Y.; Yin, R.X.; Yang, D.Z.; Nie, J. Alginate-chitosan/hydroxyapatite polyelectrolyte complex porous scaffolds: Preparation and characterization. Int. J. Biol. Macromol. 2010, 46, 199–205. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Krki, N.; Lazi, V.; Petrovi, L.; Gvozdenovi, J.; Peji, D. Properties of Chitosan-Laminated Collagen Film. Food Technol. Biotechnology 2012, 50, 483–489. [Google Scholar]
- Liu, H.; Zhao, L.; Guo, S.; Xia, Y.; Zhou, P. Modification of fish skin collagen film and absorption property of tannic acid. J. Food Sci. Technol. 2014, 51, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Wang, Y.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.; Wang, L.; Ji, P.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 95A, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, J.T.; Chen, C.L.; Huang, K.S.; Nien, Y.H.; Chen, J.L.; Huang, P.Z. Synthesis, Characterization, and Application of PVP/Chitosan Blended Polymers. J. Appl. Polym. Sci. 2006, 101, 885–891. [Google Scholar] [CrossRef]
- Lewandowska, K. Miscibility and interactions in chitosan acetate/poly (N-vinylpyrrolidone) blends. Thermochim. Acta 2011, 517, 90–97. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. The Mechanical and Biological Properties of Chitosan Scaffolds for Tissue Regeneration Templates Are Significantly Enhanced by Chitosan from Gongronella butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef] [Green Version]
- Chaiyasan, W.; Srinivas, S.P.; Tiyabooncha, W. Crosslinked chitosan-dextran sulfate nanoparticle for improved topical ocular drug delivery. Mol. Vis. 2015, 21, 1224–1234. [Google Scholar] [PubMed]
- Seol, Y.-J.; Lee, J.-Y.; Park, Y.-J.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Lee, S.-J.; Han, S.-B.; Chung, C.-P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037–1041. [Google Scholar] [CrossRef]
- Tachibana, M.; Yaita, A.; Taniura, H.; Fukasawa, K.; Nagasue, N.; Nakamura., T. The use of chitin as a new absorbable suture material-an experimental study. Jpn. J. Surg. 1988, 18, 533–539. [Google Scholar] [CrossRef]
- Bergera, J.; Reista, M.; Mayera, J.M.; Feltb, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Mane, S.; Ponrathnam, S.; Chavan, N. Effect of Chemical Cross-linking on Properties of Polymer Microbeads: A Review canchemtrans. Can. Chem. Trans. 2015, 3, 473–485. [Google Scholar]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Appl. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Chiono, V.; Pulieri, E.; Vozzi, G.; Ciardelli, G.; Ahluwalia, A.; Giusti, P. Genipin-crosslinked chitosan/gelatin blends for biomedical Applications. J. Mater. Sci. Mater. Med. 2008, 19, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Sagbas, S.; Bitlisli, B.O. p(AAm/TA)-based IPN hydrogel films with antimicrobial and antioxidant properties for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41876. [Google Scholar]
- Sionkowska, A.; Kaczmarek, B.; Gnatowska, M.; Kowalonek, J. The influence of UV-irradiation on chitosan modified by the tannic acid addition. J. Photochem. Photobiol. B Biol. 2015, 148, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Cheryl, P. Tannic Acid Crosslinked Collagens and Potential for Breast Tissue Engineering. Master’s Thesis, Clemson University, Clemson, South Carolina, 2006. [Google Scholar]
- Muzzarelli, R.A.A.; Mehtedi, M.E.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [Green Version]
- Matcham, S.; Novakovic, K. Fluorescence Imaging in Genipin Crosslinked Chitosan–Poly (vinyl pyrrolidone) Hydrogels. Polymers 2016, 8, 385. [Google Scholar] [CrossRef]
- Manickam, B.; Sreedharan, R.; Elumalai, M. ‘Genipin’—The Natural Water Soluble Cross-linking Agent and Its Importance in the Modified Drug Delivery Systems: An Overview. Curr. Drug Deliv. 2014, 11, 139–145. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part A. 2008, 87, 308–320. [Google Scholar] [CrossRef]
- Lai, J.Y. Biocompatibility of Genipin and Glutaraldehyde Cross-Linked Chitosan Materials in the Anterior Chamber of the Eye. Int. J. Mol. Sci. 2012, 13, 10970–10985. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.Y.; Li, Y.T.; Wang, T.P. In vitro response of retinal pigment epithelial cells exposed to chitosan materials prepared with different cross-linkers. Int. J. Mol. Sci. 2010, 11, 5256–5272. [Google Scholar] [CrossRef] [Green Version]
- Mi, F.L.; Tan, Y.C.; Liang, H.F.; Sung, H.W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef]
- Grolik, M.; Szczubiałka, K.; Wowra, B.; Dobrowolski, D.; Orzechowska-Wylęgała, B.; Wylęgała, E.; Nowakowska, M. Hydrogel membranes based on genipin-cross-linked chitosan blends for corneal epithelium tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 1991–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Saha, N.; Kuceková, Z.; Humpolicek, P.; Saha, P. Properties of biomineralized (CaCO3) PVP-CMC hydrogel with reference to its cytotoxicity. Int. J. Polym. Mater. 2016, 65, 619–628. [Google Scholar] [CrossRef]
- Fathi, M.; Entezami, A.A.; Pashaei-Asl, R. Swelling/deswelling, thermal, and rheological behavior of PVA-g-NIPAAm nanohydrogels prepared by a facile free-radical polymerization method. J. Polym. Res. 2013, 20, 125. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Martins, A.M.; Castelhano-Carlos, M.J.; Correlo, V.M.; Sol, P.; Longatto-Filho, A.; Battacharya, M.; Reis, R.L.; Neves, N.M. In vitro degradation and in vivo biocompatibility of chitosan–poly (butylene succinate) fiber mesh scaffolds. J. Bioact. Compat. Polym. 2014, 29, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Rejmontová, P.; Capáková, Z.; Mikušová, N.; Maráková, N.; Kašpárková, V.; Lehocký, M.; Humpolíček, P. Adhesion, proliferation and migration of NIH/3T3 cells on modified polyaniline surfaces. Int. J. Mol. Sci. 2016, 17, 1439. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.L.; Resende, C.X.; Tavares, D.S.; Soares, G.A. Cytocompatibility of Chitosan and Collagen-Chitosan Scaffolds for Tissue Engineering. Polímeros 2011, 21, 1–6. [Google Scholar] [CrossRef]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linkedcollagen scaffold and its application in wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101 Pt B, 560–567. [Google Scholar] [CrossRef]
- Dimida, S.; Demitri, C.; Benedictis, V.M.D.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015, 132, 42256. [Google Scholar] [CrossRef]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.G.; Rodrigues, R.C.; Costa, T.M.H.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and B-d-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Dimida, S.; Barca, A.; Cancelli, N.; de Benedictis, V.; Raucci, M.G.; Demitri, C. Effects of Genipin Concentration on Cross-Linked Chitosan Scaffolds for Bone Tissue Engineering: Structural Characterization and Evidence of Biocompatibility Features. Int. J. Polym. Sci. 2017, 2017, 8410750. [Google Scholar] [CrossRef] [Green Version]
- Mayra, A.P.C.; Horacio, G.R. Study by infrared spectroscopy and thermogravimetric analysis of Tannins and Tannic acid. Lat. Am. J. Chem. 2011, 39, 107–112. [Google Scholar]
- Mirzaei, E.; Majidi, R.F.; Shokrgozar, M.A.; Paskiabi, F.A. Genipin cross-linked electrospun chitosan-based nanofibrous mat as tissueengineering scaffold. Nanomed. J. 2014, 1, 137–146. [Google Scholar]
- Ma, L.; Gao, C.Y.; Mao, Z.W.; Shen, J.C.; Hu, X.Q.; Han, C.M. Thermal dehydration treatment and glutaraldehyde cross-linking to increase the biostability of collagen-chitosan porous scaffolds used as dermal equivalent. J. Biomater. Sci. Polym. Ed. 2003, 14, 861–874. [Google Scholar]
- Horn, M.M.; Martins, V.C.A.; Plepis, A.M.G. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Poly. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Physical and Chemical Treatments on Chitosan Matrix to Modify Film Properties and Kinetics of Biodegradation. Mater. Chem. Phys. 2013, 1, 51–57. [Google Scholar]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Memb. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Peña, C.; Caba, K.; Eceiza, A.; Ruseckaite, R.; Mondragon, I. Enhancing water repellence and mechanical properties of gelatin films by tannin addition. Bioresour. Technol. 2010, 101, 6836–6842. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hosseinzadeh, H. Synthesis and super-swelling behavior of a novel low salt-sensitive protein-based superabsorbent hydrogel: Collagen-g-poly(AMPS)H. Turk. J. Chem. 2010, 34, 739–752. [Google Scholar]
- Hankiewicz, J.; Swierczek, E. Lysozyme in human body fluids. Clin. Chim. Acta 1974, 57, 205–209. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Mohammadi Samani, S.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; et al. Effects of genipin cross-linking of chitosan hydrogels on cellularadhesion and viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef]







| Sample Index | Degradation of Film (%) | |
|---|---|---|
| Lysozyme | Lipase | |
| Col/Chi-Ta | 14 | 15 |
| DC-Col/Chi | 11 | 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications. Polymers 2019, 11, 2094. https://doi.org/10.3390/polym11122094
Shah R, Stodulka P, Skopalova K, Saha P. Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications. Polymers. 2019; 11(12):2094. https://doi.org/10.3390/polym11122094
Chicago/Turabian StyleShah, Rushita, Pavel Stodulka, Katerina Skopalova, and Petr Saha. 2019. "Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications" Polymers 11, no. 12: 2094. https://doi.org/10.3390/polym11122094