Pressure Field Assisted Polycondensation Nonaqueous Precipitation Synthesis of Mullite Whiskers and Their Application as Epoxy Resin Reinforcement
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Precipitate Phase Transition Analysis
3.2. Effect of Pressure Field Caused by Kettle Treatment

3.3. Effect of AlF3Amount on Sample Morphology
3.4. Structure Analysis of Whiskers
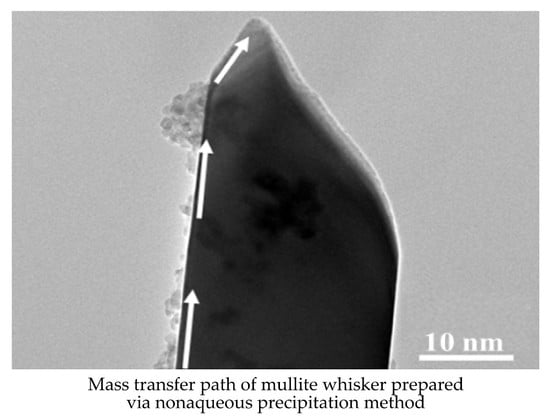
3.5. Whiskers Growth Mechanism Analysis
3.6. Application of Whiskers in Epoxy Resin Reinforcement
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, S.; Du, H. Preparation of self-reinforcement of porous mullite ceramics through in situ synthesis of mullite whisker in flyash body. Ceram. Int. 2011, 38, 1027–1032. [Google Scholar] [CrossRef]
- Xu, L.; Xi, X.; Zhu, W. Preparation of mullite whisker skeleton porous ceramic. Ceram. Int. 2015, 41, 11576–11579. [Google Scholar] [CrossRef]
- She, J.H.; Ohji, T. Fabrication and characterization of highly porous mullite ceramics. Mater. Chem. Phys. 2003, 80, 610–614. [Google Scholar] [CrossRef]
- Wang, M.; Xie, C. Polymer-derived silicon nitride ceramics by digital light processing based additive manufacturing. J. Am. Ceram. Soc. 2019, 102, 5117–5126. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Xie, C. High solid loading, low viscosity photosensitive Al2O3 slurry for stereolithography based additive manufacturing. Ceram. Int. 2019, 45, 203–208. [Google Scholar] [CrossRef]
- Kim, B.M.; Cho, Y.K. Mullite whiskers derived from kaolin. Ceram. Int. 2009, 35, 579–583. [Google Scholar] [CrossRef]
- Okada, K.; Otsuka, N. Synthesis of mullite whiskers by vapour-phase process. J. Mater. Sci. Lett. 1989, 8, 1052–1054. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, H. Synthesis of mullite whiskers using molten salt method. Rare Metal. Mat. Eng. 2009, 38, 1154–1157. [Google Scholar]
- Xiang, W.; Li, J.H. Phase evolution and dynamics of cerium-doped mullite whiskers synthesized by sol–gel process. Ceram. Int. 2013, 39, 9677–9681. [Google Scholar]
- Jiang, W.H.; Peng, Y.F. Preparation of mullite whisker via non-hydrolytic sol-gel route. J. Inorg. Mater. 2010, 25, 532–536. [Google Scholar] [CrossRef]
- Fu, K.L.; Jiang, W.H. Low temperature preparation of mullite whisker via non-hydrolytic sol-gel process combined with molten salt method. Adv. Mater. Res. 2014, 936, 975–980. [Google Scholar] [CrossRef]
- Fu, K.L.; Jiang, W.H. Study on mullite whiskers preparation via non-hydrolytic sol-gel process combined with molten salt method. Mater. Sci. Forum. 2016, 848, 295–300. [Google Scholar] [CrossRef]
- Liu, J.M.; Fu, K.L. Preparation of mullite whiskers using lithium molybdate as molten salt. Chin. Ceram. 2017, 53, 7–12. [Google Scholar]
- Wu, Q.; Jiang, W.H. Preparation of mullite whiskers by non-hydrolytic sol-gel process combined with molten salt method. Chin. Ceram. 2016, 52, 12–16. [Google Scholar]
- Feng, G.; Jiang, F. Novel facile nonaqueous precipitation in-situ synthesis of mullite whisker skeleton porous materials. Ceram. Int. 2018, 44, 22904–22910. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, W.H. Novel nonaqueous precipitation synthesis of alumina powders. Ceram. Int. 2017, 43, 13461–13468. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, F. Effect of oxygen donor alcohol on nonaqueous precipitation synthesis of alumina powders. Ceram. Int. 2019, 45, 354–360. [Google Scholar] [CrossRef]
- Vieira, S.C.; Ramos, A.S. Mullitization kinetics from silica- and alumina-rich wastes. Ceram. Int. 2007, 33, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.H.; Peng, Y.F. Low temperature synthesis of high purity mullite powder via non-hydrolytic sol-gel method. Chin. Ceram. 2010, 25, 532–536. [Google Scholar]
- Hua, K.; Xi, X. Effects of AlF3 and MoO3 on properties of mullite whisker reinforced porous ceramics fabricated from construction waste. Ceram. Int. 2016, 42, 17179–171840. [Google Scholar] [CrossRef]
- Yang, F.; Shi, Y. Analysis of whisker growth on a surface of revolution. Phys. Lett. A 2017, 381, 1–5. [Google Scholar] [CrossRef]
- Lhymn, C.; Tempelmeyer, K.E. The abrasive wear of short fibre composites. Composites 1985, 16, 127–136. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.; Jiang, F.; Hu, Z.; Jiang, W.; Liu, J.; Zhang, Q.; Hu, Q.; Miao, L.; Wu, Q.; Liang, J. Pressure Field Assisted Polycondensation Nonaqueous Precipitation Synthesis of Mullite Whiskers and Their Application as Epoxy Resin Reinforcement. Polymers 2019, 11, 2007. https://doi.org/10.3390/polym11122007
Feng G, Jiang F, Hu Z, Jiang W, Liu J, Zhang Q, Hu Q, Miao L, Wu Q, Liang J. Pressure Field Assisted Polycondensation Nonaqueous Precipitation Synthesis of Mullite Whiskers and Their Application as Epoxy Resin Reinforcement. Polymers. 2019; 11(12):2007. https://doi.org/10.3390/polym11122007
Chicago/Turabian StyleFeng, Guo, Feng Jiang, Zi Hu, Weihui Jiang, Jianmin Liu, Quan Zhang, Qing Hu, Lifeng Miao, Qian Wu, and Jian Liang. 2019. "Pressure Field Assisted Polycondensation Nonaqueous Precipitation Synthesis of Mullite Whiskers and Their Application as Epoxy Resin Reinforcement" Polymers 11, no. 12: 2007. https://doi.org/10.3390/polym11122007